Question 21 of 26 <> When 4.21 g of a nonelectrolyte solute is dissolved in water to make 805 mL of solution at 25 °C, the solution exerts an osmotic pressure of 849 torr. What is the molar concentration of the solution? concentration: How many moles of solute are in the solution? moles of solute: mol
Question 21 of 26 <> When 4.21 g of a nonelectrolyte solute is dissolved in water to make 805 mL of solution at 25 °C, the solution exerts an osmotic pressure of 849 torr. What is the molar concentration of the solution? concentration: How many moles of solute are in the solution? moles of solute: mol
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 4QAP: Solutions Introduced directly into the bloodstream have to be isotonic with blood; that is, they...
Related questions
Question
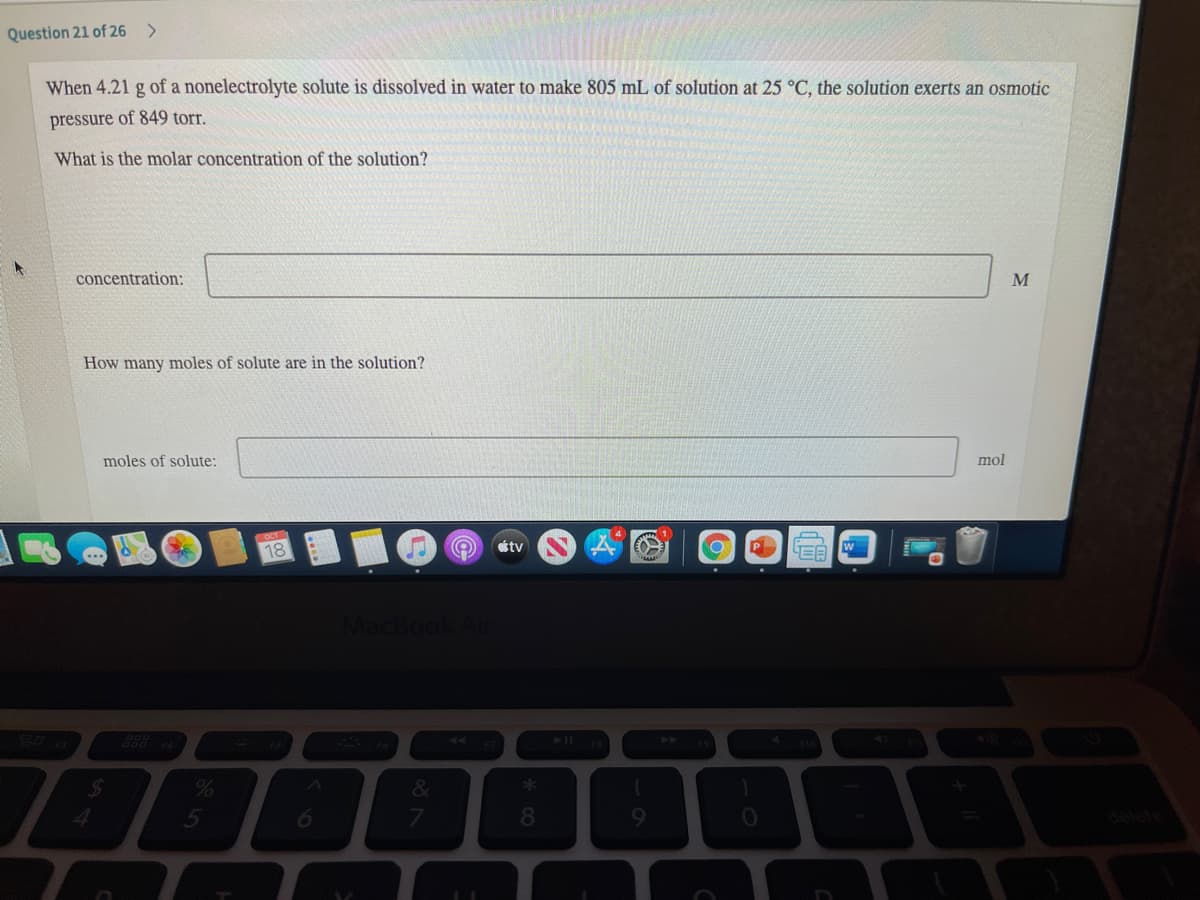
Transcribed Image Text:Question 21 of 26
<>
When 4.21 g of a nonelectrolyte solute is dissolved in water to make 805 mL of solution at 25 °C, the solution exerts an osmotic
pressure of 849 torr.
What is the molar concentration of the solution?
concentration:
M
How many moles of solute are in the solution?
moles of solute:
mol
otv
P
Book
Il FB
*
08.
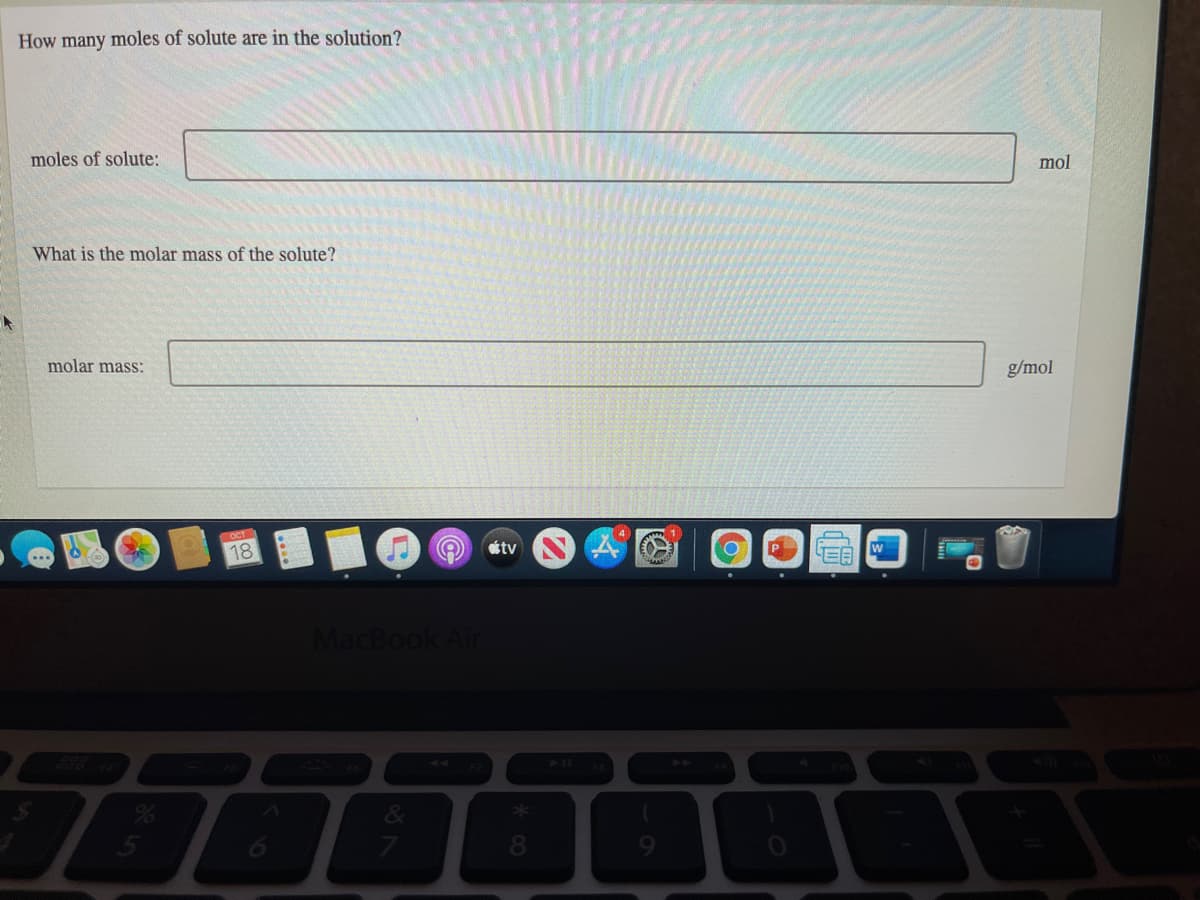
Transcribed Image Text:How many moles of solute are in the solution?
moles of solute:
mol
What is the molar mass of the solute?
molar mass:
g/mol
18
tv
MacBook
*
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning