QUESTION 8 An alternative way to prepare aspirin (an ester) is to react acetic acid (a carboxylic acid) with salicylic acid (an alcohol), in the presence of H 3PO 4. This reaction is shown at the top of the diagram below. In addition to aspirin, water forms as a product. The water is formed from the portions of the reactant molecules that are enclosed in the box in the diagram. One of the aspects of organic chemistry that makes it incredibly useful is the fact that the reactions characteristic of each functional group will happen with ANY molecule that contains that functional group! ANY carboxylic acid can react with ANY alcohol (in the presence of an inorganic acid like H 3PO 4) to yield an ester. Ethyl butyrate is an ester that has the odor of pineapples (it is often used as "artificial" pineapple flavoring in candy). It can be prepared by reacting a carboxylic acid with an alcohol in the presence of acid. What is the structure of the carboxylic acid that must be involved in the synthesis of ethyl butyrate? H₂C carboxylic acid ??? FOH + HO (Question 8) CH3CH₂CH₂ OH HO CH3CH₂ OH CH3CH₂OH CH3CH₂CH₂OH H alcohol ??? (Question 9) H H₂PO4 acid HO O=C CH3 H ester H C-H + H₂O CH3CH₂CH₂ OCH₂CH3 Ethyl butyrate (pineapple odor)
QUESTION 8 An alternative way to prepare aspirin (an ester) is to react acetic acid (a carboxylic acid) with salicylic acid (an alcohol), in the presence of H 3PO 4. This reaction is shown at the top of the diagram below. In addition to aspirin, water forms as a product. The water is formed from the portions of the reactant molecules that are enclosed in the box in the diagram. One of the aspects of organic chemistry that makes it incredibly useful is the fact that the reactions characteristic of each functional group will happen with ANY molecule that contains that functional group! ANY carboxylic acid can react with ANY alcohol (in the presence of an inorganic acid like H 3PO 4) to yield an ester. Ethyl butyrate is an ester that has the odor of pineapples (it is often used as "artificial" pineapple flavoring in candy). It can be prepared by reacting a carboxylic acid with an alcohol in the presence of acid. What is the structure of the carboxylic acid that must be involved in the synthesis of ethyl butyrate? H₂C carboxylic acid ??? FOH + HO (Question 8) CH3CH₂CH₂ OH HO CH3CH₂ OH CH3CH₂OH CH3CH₂CH₂OH H alcohol ??? (Question 9) H H₂PO4 acid HO O=C CH3 H ester H C-H + H₂O CH3CH₂CH₂ OCH₂CH3 Ethyl butyrate (pineapple odor)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.83PAE
Related questions
Question
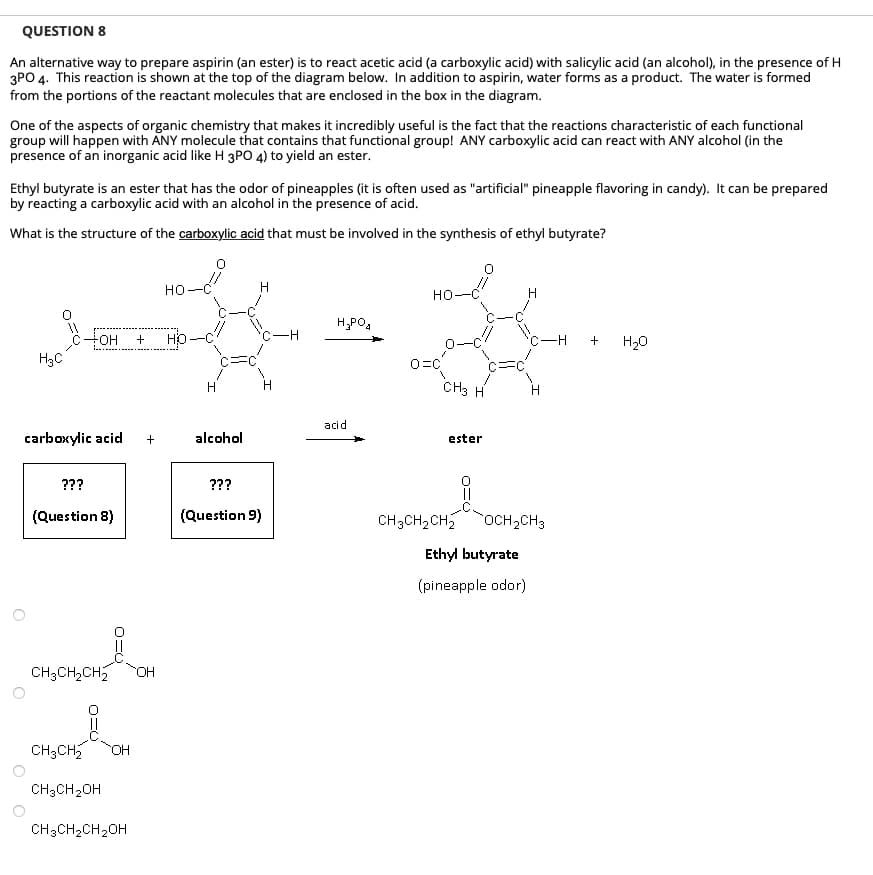
Transcribed Image Text:QUESTION 8
An alternative way to prepare aspirin (an ester) is to react acetic acid (a carboxylic acid) with salicylic acid (an alcohol), in the presence of H
3PO 4. This reaction is shown at the top of the diagram below. In addition to aspirin, water forms as a product. The water is formed
from the portions of the reactant molecules that are enclosed in the box in the diagram.
One of the aspects of organic chemistry that makes it incredibly useful is the fact that the reactions characteristic of each functional
group will happen with ANY molecule that contains that functional group! ANY carboxylic acid can react with ANY alcohol (in the
presence of an inorganic acid like H 3PO 4) to yield an ester.
Ethyl butyrate is an ester that has the odor of pineapples (it is often used as "artificial" pineapple flavoring in candy). It can be prepared
by reacting a carboxylic acid with an alcohol in the presence of acid.
What is the structure of the carboxylic acid that must be involved in the synthesis of ethyl butyrate?
H3C
FOH + HO
carboxylic acid
???
(Question 8)
CH3CH₂CH₂ OH
CH3CH₂ OH
CH3CH₂OH
+
CH3CH₂CH₂OH
HO
c=
alcohol
???
(Question 9)
H₂PO4
acid
но
O=C
CH3 H
ester
ܘ
—H +
CH3CH₂CH₂ OCH₂CH3
Ethyl butyrate
(pineapple odor)
H₂O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning