QUESTION 8 if 192.5 ml He balloon rises in altitude from sea-level where the pressure is 1.00 atm to 759ft. where the pressures is 748 torr, what is the volume of the balloon at this altitude? Round your answer to 1decimal place and be sure to use the correct abbreviation for the UNITS
QUESTION 8 if 192.5 ml He balloon rises in altitude from sea-level where the pressure is 1.00 atm to 759ft. where the pressures is 748 torr, what is the volume of the balloon at this altitude? Round your answer to 1decimal place and be sure to use the correct abbreviation for the UNITS
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter20: Molecular Mass Spectrometry
Section: Chapter Questions
Problem 20.19QAP
Related questions
Question
100%
Transcribed Image Text:G what shows that there
Content
Take Test: HW- 15 Boy x
A Reacting one mole of
Q How many electrons d X
O Chem I Homework Ex x
A Microsoft Word-CHO X
G al charge-Google Se x
->
->
A blackboard.matc.edu/webapps/assessment/take/launch.jsp?course assessment_id3_713181 1&course_id=_496734 1&content_id=_11528137 1&step-null
* Question Completion Status:
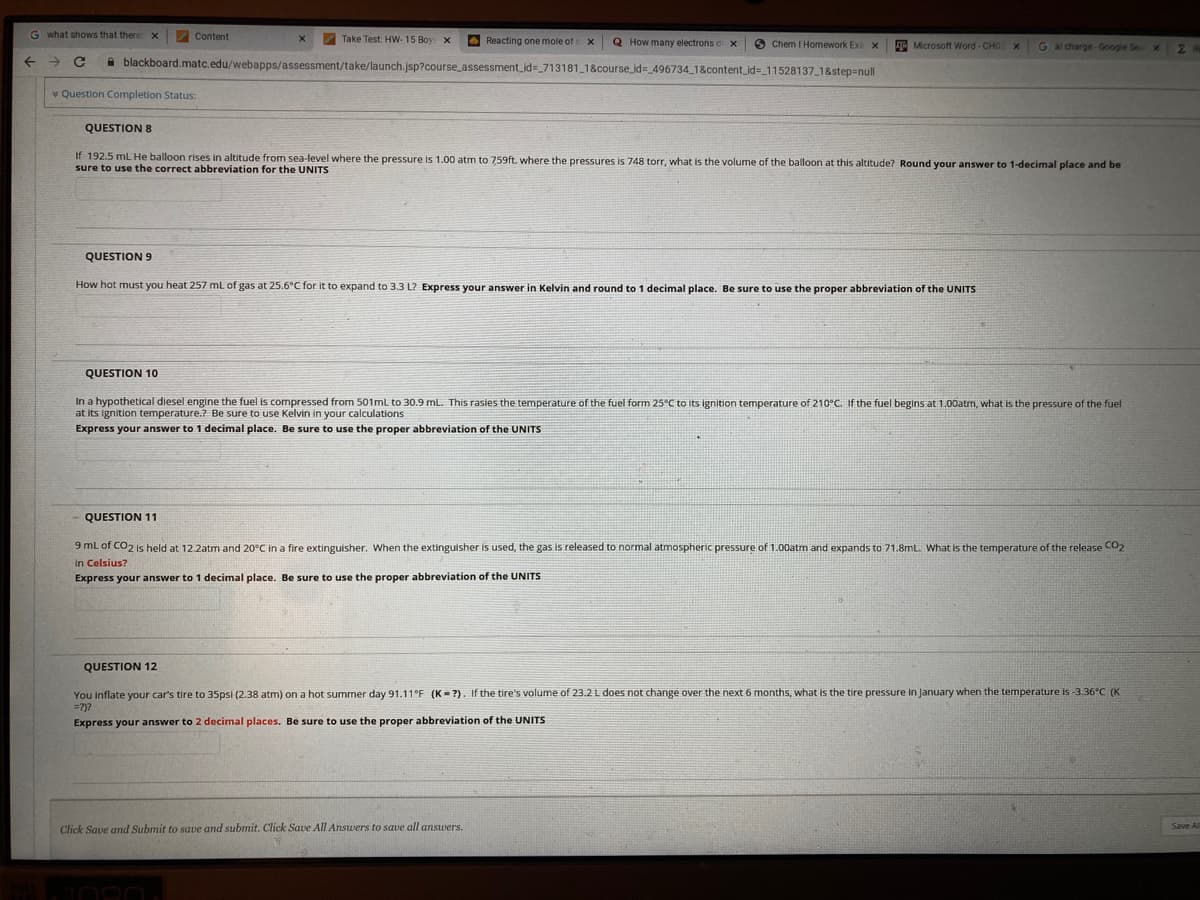
QUESTION 8
If 192.5 mL He balloon rises in altitude from sea-level where the pressure is 1.00 atm to 759ft. where the pressures is 748 torr, what is the volume of the balloon at this altitude? Round your answer to 1-decimal place and be
sure to use the correct abbreviation for the UNITS
QUESTION 9
How hot must you heat 257 mL of gas at 25.6°C for it to expand to 3.3 L? Express your answer in Kelvin and round to 1 decimal place. Be sure to use the proper abbreviation of the UNITS
QUESTION 10
In a hypothetical diesel engine the fuel is compressed from 501ml to 30.9 mL. This rasies the temperature of the fuel form 25°C to its ignition temperature of 210°C. If the fuel begins at 1.00atm, what is the pressure of the fuel
at its ignition temperature.? Be sure to use Kelvin in your calculations
Express your answer to 1 decimal place. Be sure to use the proper abbreviation of the UNITS
QUESTION 11
9 mL of CO2 is held at 12.2atm and 20°C in a fire extinguisher. When the extinguisher is used, the gas is released to normal atmospheric pressure of 1.00atm and expands to 71.8mL. What is the temperature of the release CO2
in Celsius?
Express your answer to 1 decimal place. Be sure to use the proper abbreviation of the UNITS
QUESTION 12
You Inflate your car's tire to 35psi (2.38 atm) on a hot summer day 91.11°F (K = ?). If the tire's volume of 23.2L does not change over the next 6 months, what is the tire pressure in January when the temperature is -3.36°C (K
=?)?
Express your answer to 2 decimal places. Be sure to use the proper abbreviation of the UNITS
Save Al
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
