QUESTION 8 Three titration trials were performed and the concentration of NaOH was determined for each trial. The concentrations were determined as follows: 0.19 M 0.12 M 0.12 M Calculate the average molar concentration of NaOH. QUESTION 9 The average concentration of NaOH for all titrations was determined to be 0.13, but the true concentration is 0.10. Calculate the percent error for the experiment.
QUESTION 8 Three titration trials were performed and the concentration of NaOH was determined for each trial. The concentrations were determined as follows: 0.19 M 0.12 M 0.12 M Calculate the average molar concentration of NaOH. QUESTION 9 The average concentration of NaOH for all titrations was determined to be 0.13, but the true concentration is 0.10. Calculate the percent error for the experiment.
Chapter8: Polyfunctional Acids And Bases
Section: Chapter Questions
Problem 10P
Related questions
Question
8 and 9
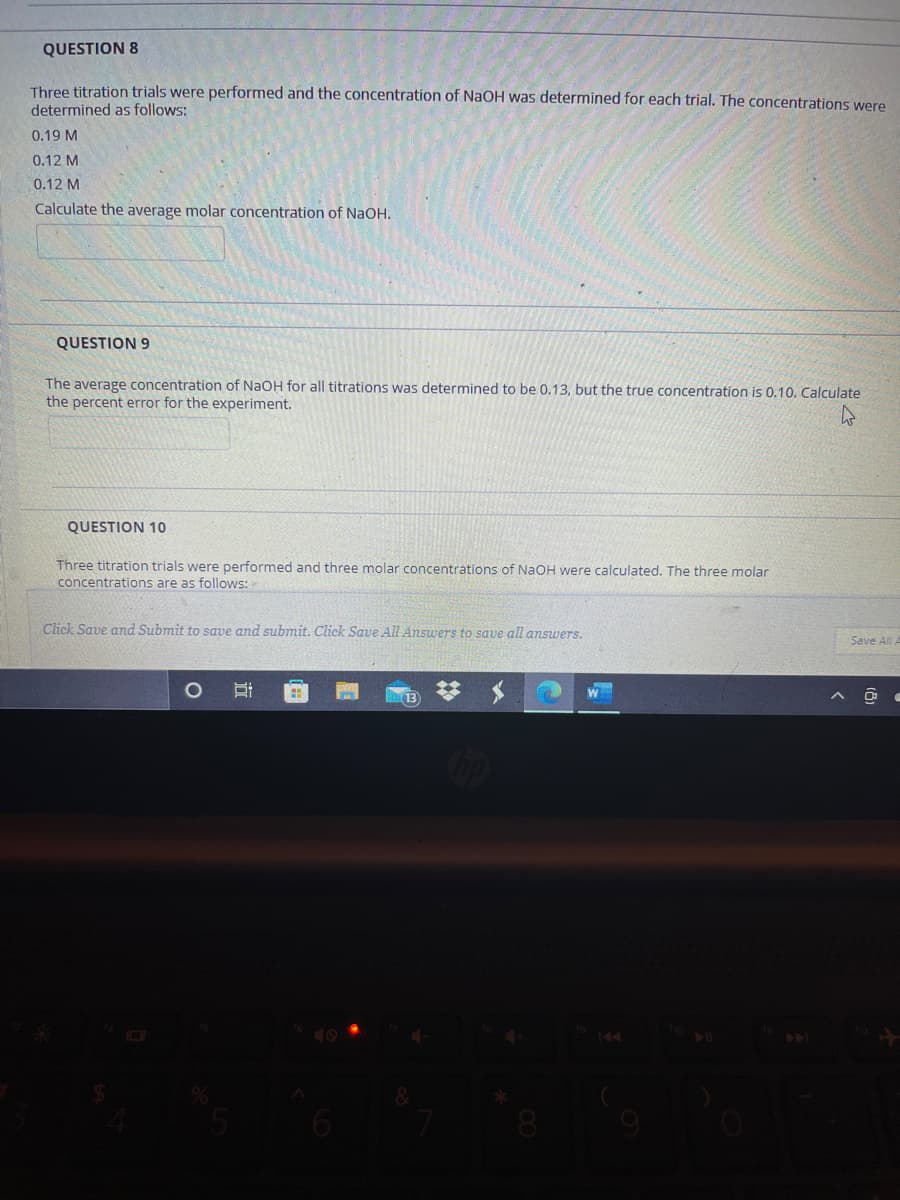
Transcribed Image Text:QUESTION 8
Three titration trials were performed and the concentration of NaOH was determined for each trial. The concentrations were
determined as follows:
0.19 M
0.12 M
0.12 M
Calculate the average molar concentration of NaOH.
QUESTION 9
The average concentration of NaOH for all titrations was determined to be 0.13, but the true concentration is 0.10. Calculate
the percent error for the experiment.
QUESTION 10
Three titration trials were performed and three molar concentrations of NaOH were calculated. The three molar
concentrations are as follows:
Click Save and Submit to save and submit. Click Save All Answers to save all ansuwers.
Save All A
40
144
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



