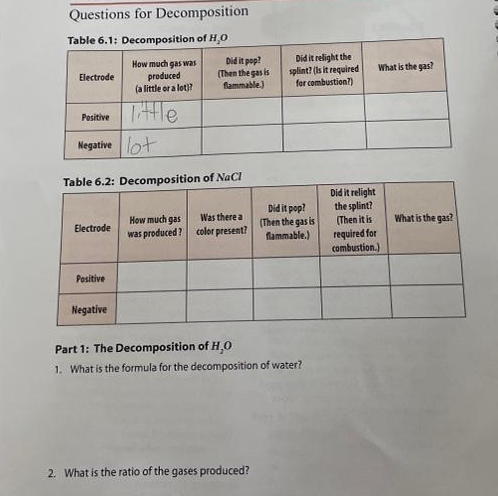
Questions for Decomposition Table 6.1: Decomposition of H₂O How much gas was produced (a little or a lot)? Electrode Electrode Positive Negative lot Table 6.2: Decomposition of NaCl Positive Tittle Negative Did it pop? (Then the gas is flammable.) Was there a How much gas was produced? color present? Did it relight the splint? (Is it required. for combustion?) 2. What is the ratio of the gases produced? Did it pop? (Then the gas is flammable.) Part 1: The Decomposition of H,O 1. What is the formula for the decomposition of water? What is the gas? Did it relight the splint? (Then it is required for combustion.) What is the gas?
Science behind corrosion-test
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
Corrosion
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 5 images









