quizzes/2332375/take O 5.12 mL HBr solution Question 4 8 p A balloon is filled so that its volume is 1165 mL when the pressure is 725 torr and the temperature is 28.5°C. What is the temperature (in °C) if it moves to a new environment where the pressure is 0.845 atm and its volume is 1.24 L? O287 °C .0.374 C 27 °C 0 11°C O 0.284 °C 8p Question 5 A 4.85 g sample of a gas is under 789 mm Hg pressure at 35.4 °C in a 2.125 L vessel. What is the molar mass of the gas?
quizzes/2332375/take O 5.12 mL HBr solution Question 4 8 p A balloon is filled so that its volume is 1165 mL when the pressure is 725 torr and the temperature is 28.5°C. What is the temperature (in °C) if it moves to a new environment where the pressure is 0.845 atm and its volume is 1.24 L? O287 °C .0.374 C 27 °C 0 11°C O 0.284 °C 8p Question 5 A 4.85 g sample of a gas is under 789 mm Hg pressure at 35.4 °C in a 2.125 L vessel. What is the molar mass of the gas?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter10: Gases And Their Properties
Section10.2: Gas Laws: The Experimental Basis
Problem 10.4CYU: You have a 22-L cylinder of helium at a pressure of 150 atm (above atmospheric pressure) and at 31...
Related questions
Question
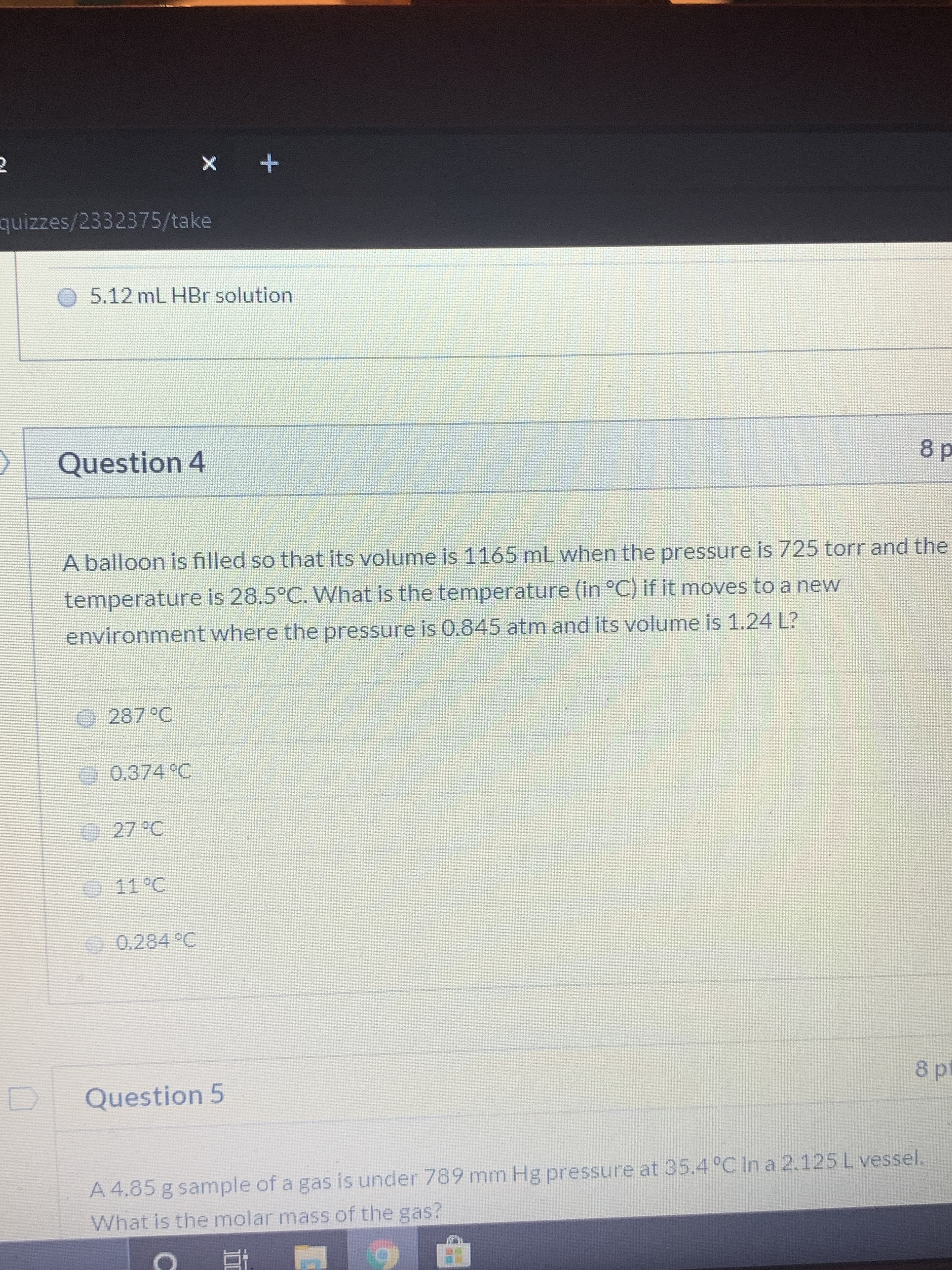
Transcribed Image Text:quizzes/2332375/take
O 5.12 mL HBr solution
Question 4
8 p
A balloon is filled so that its volume is 1165 mL when the pressure is 725 torr and the
temperature is 28.5°C. What is the temperature (in °C) if it moves to a new
environment where the pressure is 0.845 atm and its volume is 1.24 L?
O287 °C
.0.374 C
27 °C
0
11°C
O 0.284 °C
8p
Question 5
A 4.85 g sample of a gas is under 789 mm Hg pressure at 35.4 °C in a 2.125 L vessel.
What is the molar mass of the gas?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning