Radical Mono-Chlorination of pentane is a poor way to prepare 1-chloropentane, but radical chlorination of neopentane, (CH3)4C, is a good way to prepare neopentyl chloride. Write an equation for both of these reactions and explain why this is. Pentane Neopentane
Radical Mono-Chlorination of pentane is a poor way to prepare 1-chloropentane, but radical chlorination of neopentane, (CH3)4C, is a good way to prepare neopentyl chloride. Write an equation for both of these reactions and explain why this is. Pentane Neopentane
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter8: Haloalkanes, Halogenation, And Radical Reactions
Section: Chapter Questions
Problem 8.18P
Related questions
Question
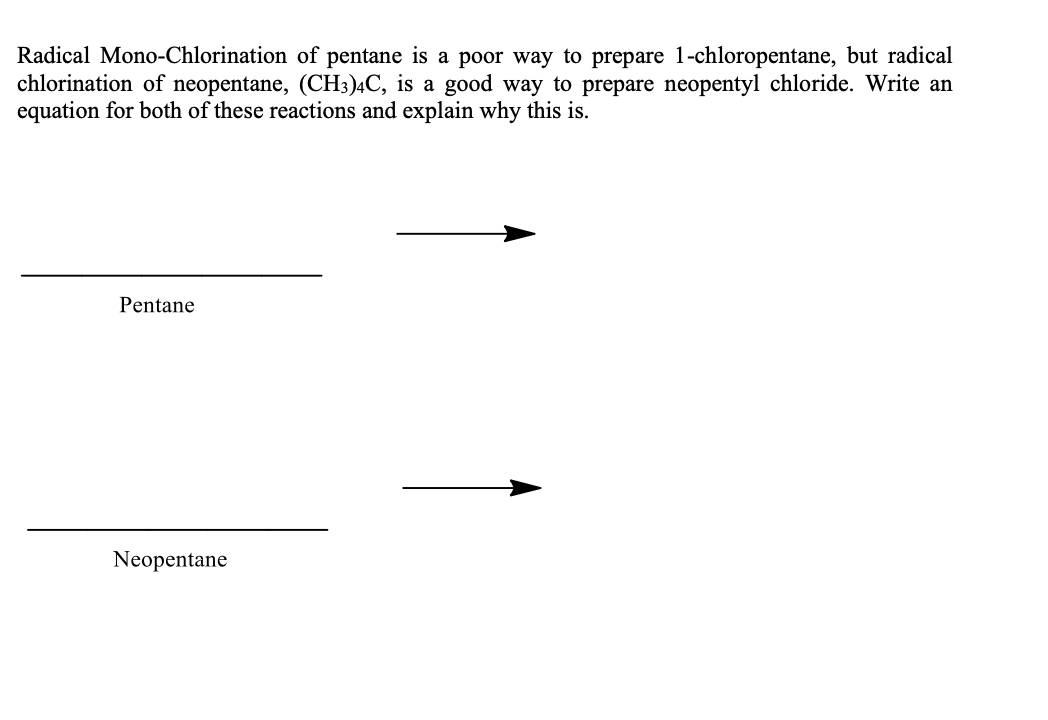
Transcribed Image Text:Radical Mono-Chlorination of pentane is a poor way to prepare 1-chloropentane, but radical
chlorination of neopentane, (CH3)4C, is a good way to prepare neopentyl chloride. Write an
equation for both of these reactions and explain why this is.
Pentane
Neopentane
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 6 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning