Chapter21: Potentiometry
Section: Chapter Questions
Problem 21.22QAP
Related questions
Question
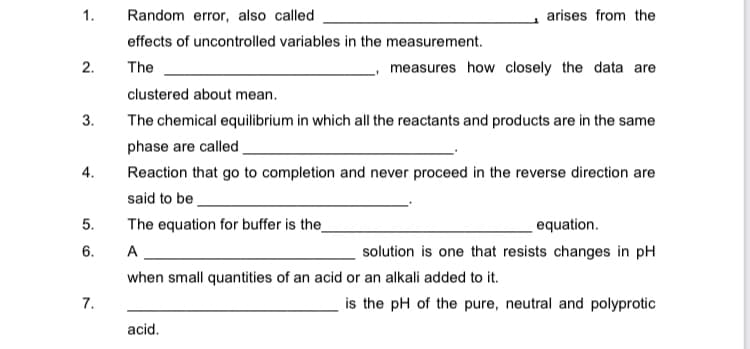
Transcribed Image Text:1.
Random error, also called
arises from the
effects of uncontrolled variables in the measurement.
2.
The
measures how closely the data are
clustered about mean.
3.
The chemical equilibrium in which all the reactants and products are in the same
phase are called.
4.
Reaction that go to completion and never proceed in the reverse direction are
said to be
5.
The equation for buffer is the_
equation.
6.
A
solution is one that resists changes in pH
when small quantities of an acid or an alkali added to it.
7.
is the pH of the pure, neutral and polyprotic
acid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax