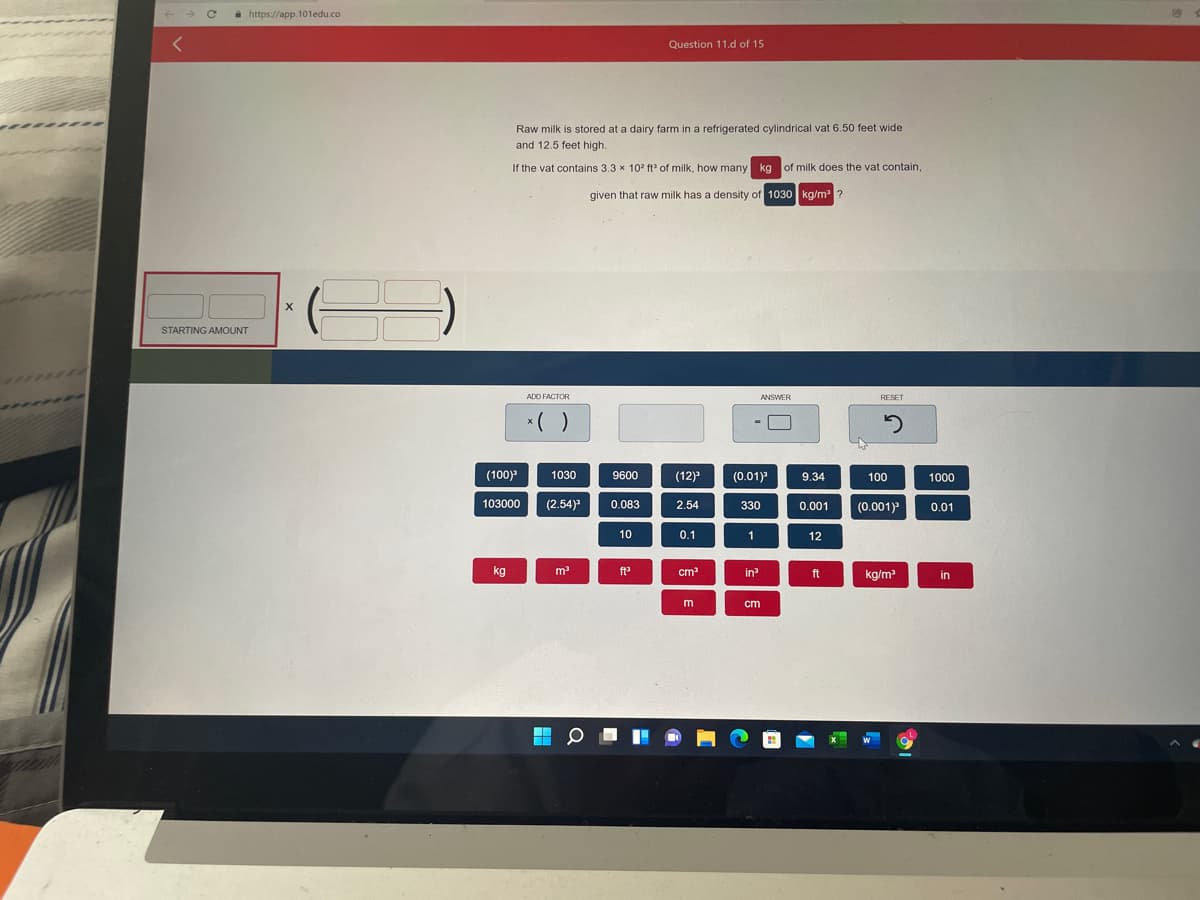
Raw milk is stored at a dairy farm in a refrigerated cylindrical vat 6.50 feet wide and 12.5 feet high. If the vat contains 3.3 x 10° ft of milk, how many kg of milk does the vat contain, given that raw milk has a density of 1030 kg/m ?
Raw milk is stored at a dairy farm in a refrigerated cylindrical vat 6.50 feet wide and 12.5 feet high. If the vat contains 3.3 x 10° ft of milk, how many kg of milk does the vat contain, given that raw milk has a density of 1030 kg/m ?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.68QE
Related questions
Question
I need to use the numbers from the bottom to answer the question
Transcribed Image Text:i https://app.101edu.co
Question 11.d of 15
Raw milk is stored at a dairy farm in a refrigerated cylindrical vat 6.50 feet wide
and 12.5 feet high.
If the vat contains 3.3 x 10? ft of milk, how many kg of milk does the vat contain,
given that raw milk has a density of 1030 kg/m ?
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
(100)
(0.01)
1030
9600
(12)
9.34
100
1000
103000
(2.54)
0.083
2.54
330
(0.001)
0.001
0.01
10
0.1
1
12
kg
m
ft
cm
in?
ft
kg/m
in
m
cm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning