re of the reaction mixture will de ur spontancously at 298 K and 1. mperature is raised, Key for this re on has more moles of products th reactants.
re of the reaction mixture will de ur spontancously at 298 K and 1. mperature is raised, Key for this re on has more moles of products th reactants.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter17: Chemcial Thermodynamics
Section: Chapter Questions
Problem 17.94QE: Suppose you have an endothermic reaction with H = + 15 kJ and a S of 150 J/K. Calculate G and Keq at...
Related questions
Question
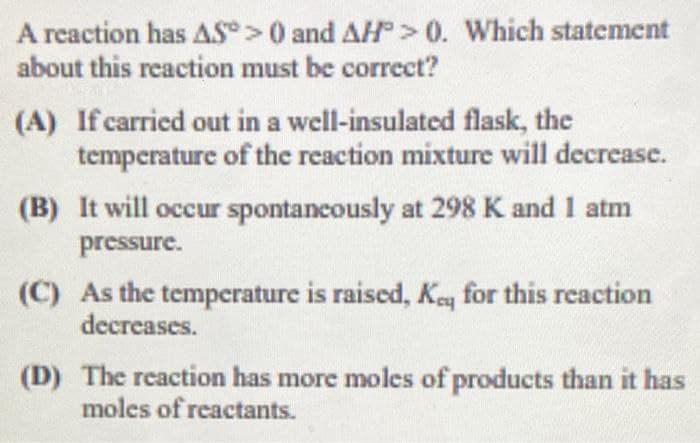
Transcribed Image Text:A reaction has AS>0 and AH>0. Which statement
about this reaction must be correct?
(A) If carried out in a well-insulated flask, the
temperature of the reaction mixture will decrease.
(B) It will occur spontancously at 298 K and 1 atm
pressure.
(C) As the temperature is raised, Keq for this reaction
decreases.
(D) The reaction has more moles of products than it has
moles of reactants.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning