Refer to Table 2, test solution 3 in the procedure. At equilibrium, the concentration of the FeNCS2+ is determined to be 1.5 x 10-4 M in 10.0 mL total volume of solution. a. Calculate the number of moles of Fe3+ and SCN- added. b. Calculate the number of moles of FeSCN2+ formed. c. Calculate the number of moles of each reactant present at equilibrium. moles at equilibrium = initial moles - moles used up d. Calculate the concentrations of all species at equilibrium. e. Calculate K.
Refer to Table 2, test solution 3 in the procedure. At equilibrium, the concentration of the FeNCS2+ is determined to be 1.5 x 10-4 M in 10.0 mL total volume of solution. a. Calculate the number of moles of Fe3+ and SCN- added. b. Calculate the number of moles of FeSCN2+ formed. c. Calculate the number of moles of each reactant present at equilibrium. moles at equilibrium = initial moles - moles used up d. Calculate the concentrations of all species at equilibrium. e. Calculate K.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter13: An Introduction To Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 13.11QAP: The equilibrium constant for the conjugate acid-base pair HIn+H2OH3O++In is 8.00 10-5. From the...
Related questions
Question
Refer to Table 2, test solution 3 in the procedure. At equilibrium, the concentration of the FeNCS2+ is determined to be 1.5 x 10-4 M in 10.0 mL total volume of solution.
a. Calculate the number of moles of Fe3+ and SCN- added.
b. Calculate the number of moles of FeSCN2+ formed.
c. Calculate the number of moles of each reactant present at equilibrium. moles at equilibrium = initial moles - moles used up
d. Calculate the concentrations of all species at equilibrium.
e. Calculate K.
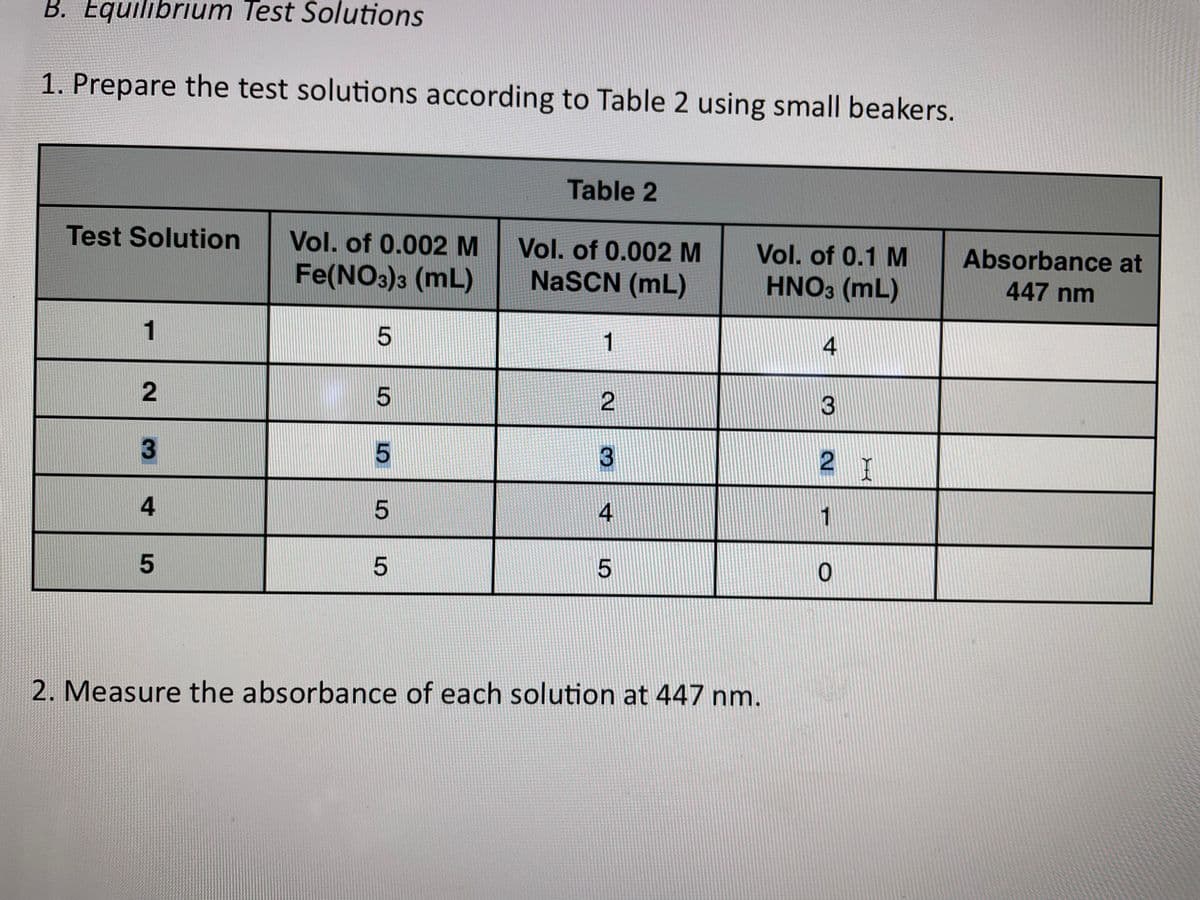
Transcribed Image Text:B. Equilibrium Test Solutions
1. Prepare the test solutions according to Table 2 using small beakers.
Table 2
Test Solution
Vol. of 0.002 M
Vol. of 0.002 M
Vol. of 0.1 M
Absorbance at
Fe(NO3)3 (mL)
NaSCN (mL)
HNO3 (mL)
447 nm
1
1
4
3
3
3
2 T
4
4
1
2. Measure the absorbance of each solution at 447 nm.
5
2.
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
