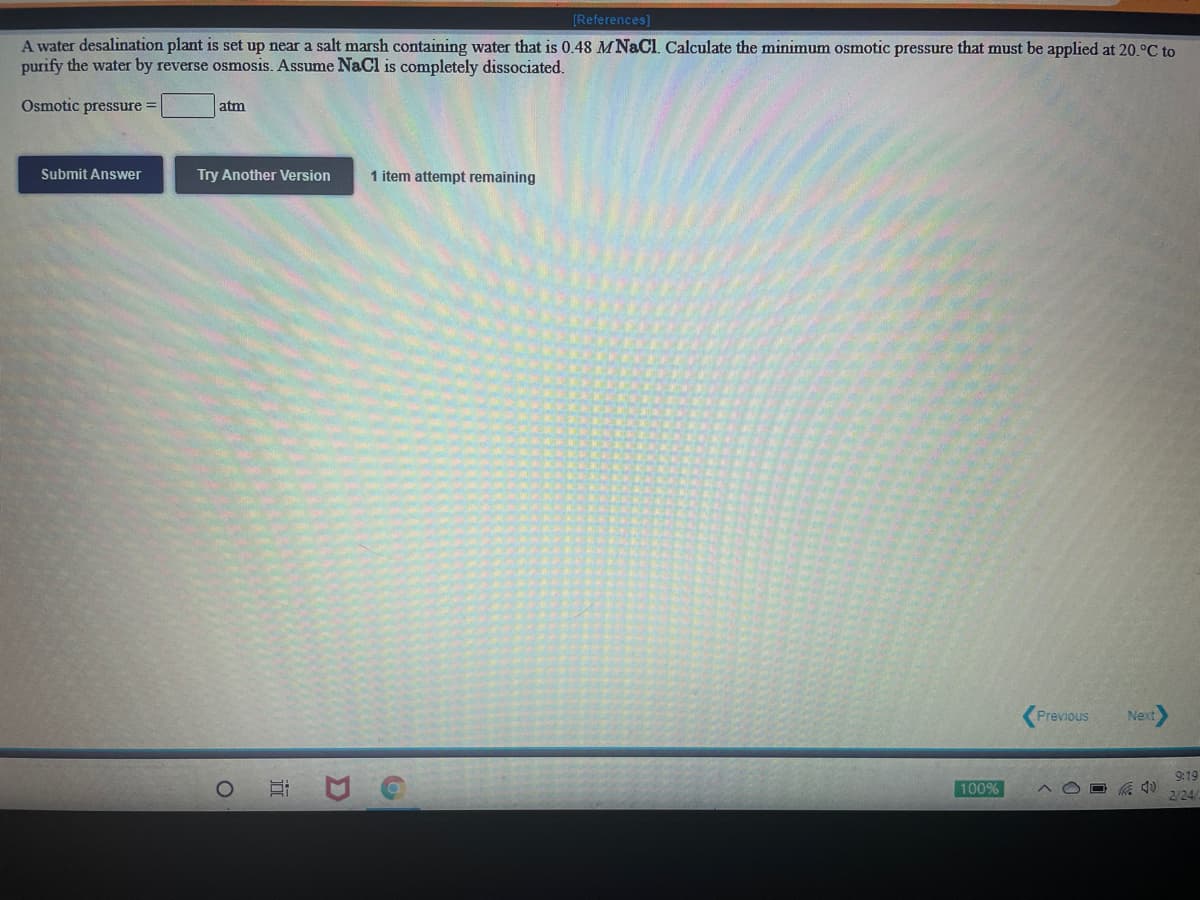
[References) A water desalination plant is set up near a salt marsh containing water that is 0.48 MNACI. Calculate the minimum osmotic pressure that must be applied at 20.°C to purify the water by reverse osmosis. Assume NaCl is completely dissociated. Osmotic pressure atm Submit Answer Try Another Version 1 item attempt remaining
[References) A water desalination plant is set up near a salt marsh containing water that is 0.48 MNACI. Calculate the minimum osmotic pressure that must be applied at 20.°C to purify the water by reverse osmosis. Assume NaCl is completely dissociated. Osmotic pressure atm Submit Answer Try Another Version 1 item attempt remaining
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 51QAP: A biochemist isolates a new protein and determines its molar mass by osmotic pressure measurements....
Related questions
Question
Transcribed Image Text:[References)
A water desalination plant is set up near a salt marsh containing water that is 0.48 MNACI. Calculate the minimum osmotic pressure that must be applied at 20.°C to
purify the water by reverse osmosis. Assume NaCl is completely dissociated.
Osmotic pressure
atm
Submit Answer
Try Another Version
1 item attempt remaining
Previous
Next
9:19
100%
2/24
近
Expert Solution
Step 1osmotic pressure
osmotic pressure is defined as pressure at which inward flow of liquid through semipermeable membrane is stopped .
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co