result? Why? 4. What is the minimum amount of ice at 0°C that must be added to the contents of a can of diet cola (340mL) to cool it down from 20.5°C to 0°C? Assume that the specific heat and density of the diet cola are the same as for water and that no heat is gained or lost to the surroundings. The latent heat of fusion of ice is 335J/g. Specific Heat of water 4.184 J/g.K, and Density of water is 1 g/mL.
result? Why? 4. What is the minimum amount of ice at 0°C that must be added to the contents of a can of diet cola (340mL) to cool it down from 20.5°C to 0°C? Assume that the specific heat and density of the diet cola are the same as for water and that no heat is gained or lost to the surroundings. The latent heat of fusion of ice is 335J/g. Specific Heat of water 4.184 J/g.K, and Density of water is 1 g/mL.
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter5: Analysis Of Convection Heat Transfer
Section: Chapter Questions
Problem 5.39P
Related questions
Question
What is the minimum amount of ice at OPC that must be added to the contents of a can of diet cola (340m) to cool it down from 20.5C to 0C Assume that the specific heat and density of the diet colo are the same as for water and that no heat is gained or lost to the surroundings. The latent heat of fusion of ice à 3353/g Specific Heat of water 4.184 J/gK and Density of water is 1 g/mL
Transcribed Image Text:4G+
5:22
个
MODULE 1_EnggChe...
27
Property of and fo the exclu
meand, electronc, mechanical photooopyng recondeg sthenwes
Prepared by: S.LTipayno, CC.Damaguen Jr
Reproduction song
d system, dsbuting, uploading or posting online. or transmitting in any form or by any
ten permission of SLU, is strictly pronibited
part
dooument without
Saint Louis University
SCHOOL OF ENGINEERING AND ARCHITECTURE
Department of Chemical Engineering
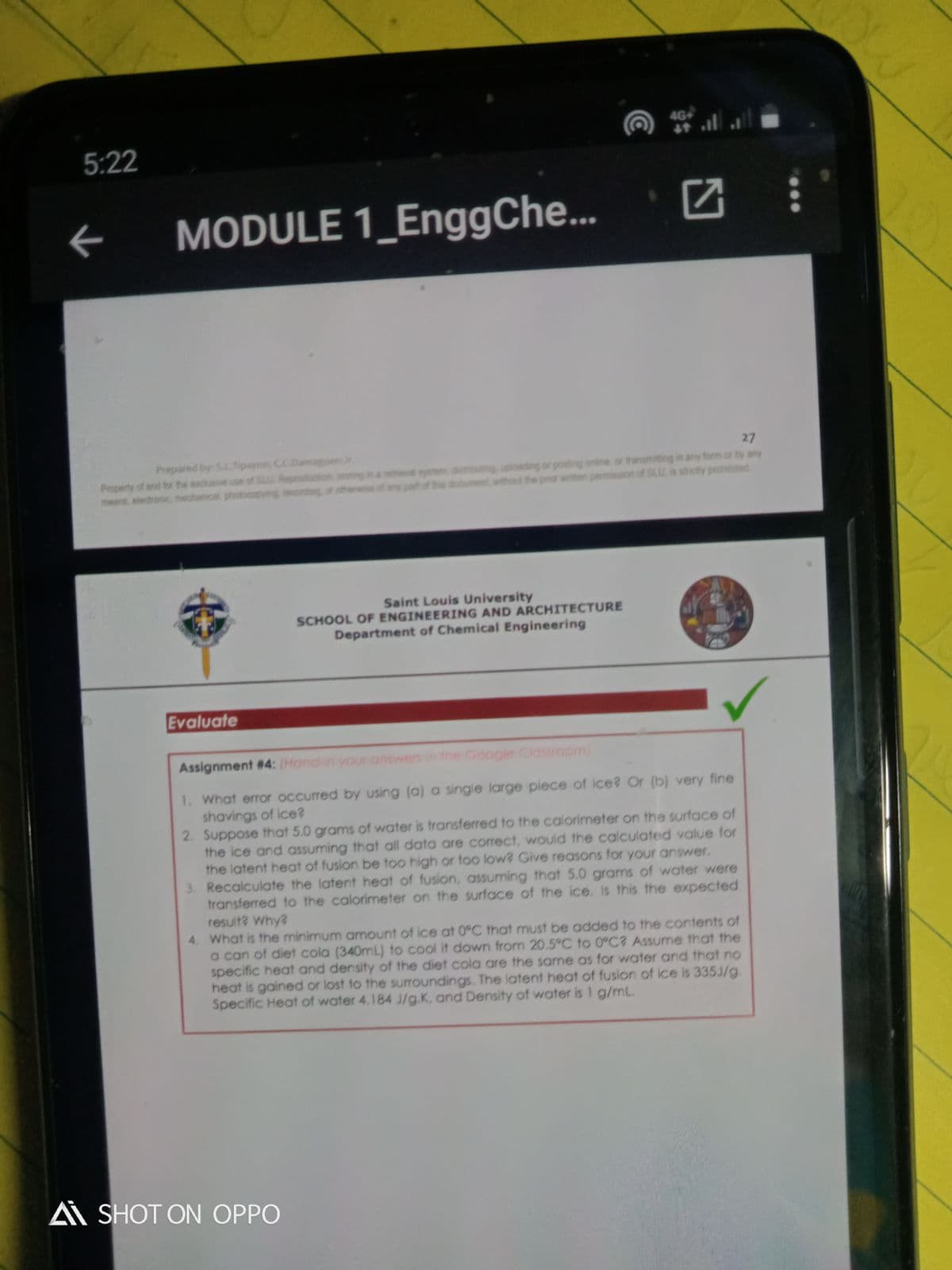
Evaluate
Assignment #4: (Hond-in your answers in the Google Classroom)
1. What error occurred by using (a) a single large piece of ice? Or (b) very fine
shavings of ice?
2. Suppose that 5.0 grams of water is transferred to the calorimeter on the surface of
the ice and assuming that all data are correct, would the calculated value for
the latent heat of fusion be too high or too low? Give reasons for your answer.
3. Recalculate the latent heat of fusion, assuming that 5.0 grams of water were
transferred to the calorimeter on the surface of the ice. Is this the expected
resuit? Why?
4. What is the minimum amount of ice at 0°C that must be added to the contents of
a can of diet cola (340mL) to cool it down from 20.5°C to 0°C? Assume that the
specific heat and density of the diet cola are the same as for water and that no
heat is gained or lost to the surroundings. The latent heat of fusion of ice is 335J/g.
Specific Heat of water 4.184 J/g.K, and Density of water is I g/mL.
A SHOT ON OPPO
Transcribed Image Text:5:28
MODULE 1_EnggChe..
27
Prepared by: S.LTipayno, C.C.Damaguen Jr.
Property of and for the exclusive use of SLU Reproduction, storing
means, electronic, mechanical, photocopying, recording, or otherwise of any part of this document, without the prior writlen permission of SLU, is strictly prohibited
retneval system, distributing, uploading or posting online, or transmitting in any form or by any
Saint Louis University
SCHOOL OF ENGINEERING AND ARCHITECTURE
Department of Chemical Eng
Ing
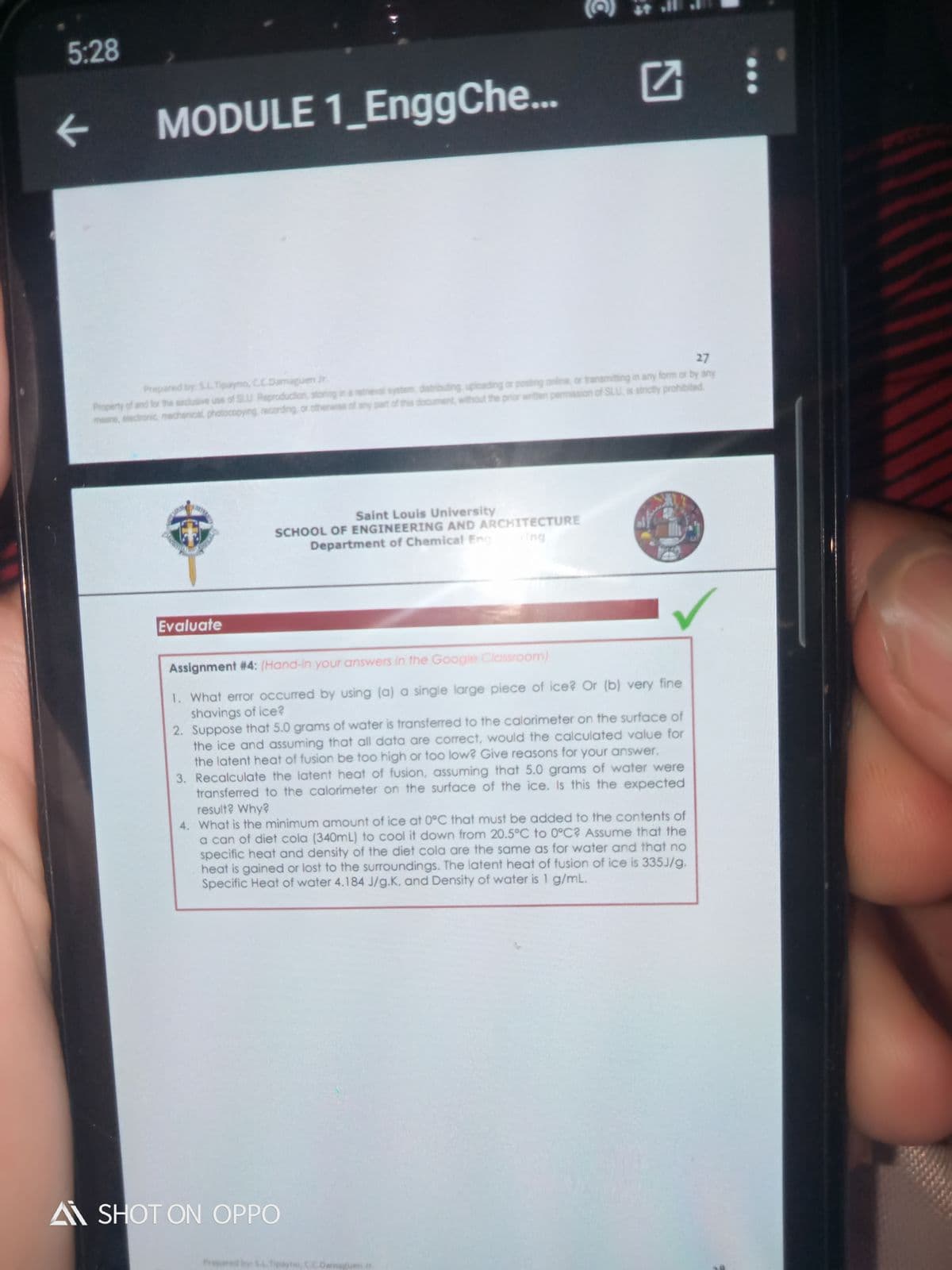
Evaluate
Assignment #4: (Hand-in your answers in the Google Classroom)
1. What error occurred by using (a) a single large piece of ice? Or (b) very fine
shavings of ice?
2. Suppose that 5.0 grams of water is transferred to the calorimeter on the surface of
the ice and assuming that all data are correct, would the calculated value for
the latent heat of fusion be too high or too low? Give reasons for your answer.
3. Recalculate the latent heat of fusion, assuming that 5.0 grams of water were
transferred to the calorimeter on the surface of the ice. Is this the expected
result? Why?
4. What is the minimum amount of ice at 0°C that must be added to the contents of
a can of diet cola (340mL) to cool it down from 20.5°C to 0°C? Assume that the
specific heat and density of the diet cola are the same as for water and that no
heat is gained or lost to the surroundings. The latent heat of fusion of ice is 335J/g.
Specific Heat of water 4.184 J/g.K, and Density of water is 1 g/mL.
A SHOT ON OPPO
Prepared by SAL Tipayno, C.C.Dam
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning