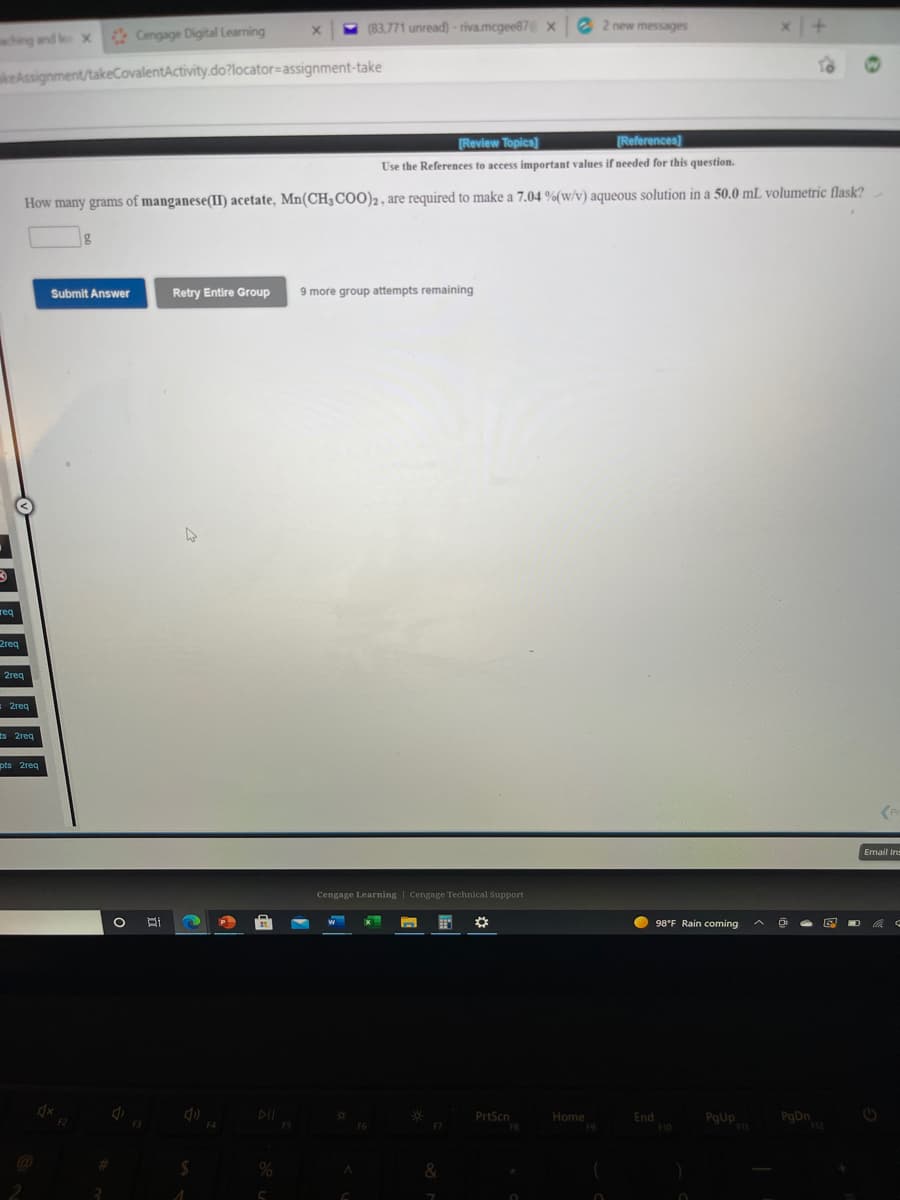
[Review Topics] [References) Use the References to access important values if needed for this question. How many grams of manganese(II) acetate, Mn(CH3C00)2, are required to make a 7.04 %(w/v) aqueous solution in a 50.0 mL volumetric flask? Retry Entire Group 9 more group attempts remaining Submit Answer
[Review Topics] [References) Use the References to access important values if needed for this question. How many grams of manganese(II) acetate, Mn(CH3C00)2, are required to make a 7.04 %(w/v) aqueous solution in a 50.0 mL volumetric flask? Retry Entire Group 9 more group attempts remaining Submit Answer
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter3: Chemical Reactions
Section3.9: Classifying Reactions In Aqueous Solution
Problem 2.2ACP
Related questions
Question
Transcribed Image Text:(83,771 unread)-riva.mcgee87 x
2 2 new messages
ing and le x Cengage Digital Learning
kekssignment/takeCovalentActivity.do?locator=assignment-take
[Review Topics)
(References)
Use the References to access important values if needed for this question.
How many grams of manganese(II) acetate, Mn(CH3COO)2, are required to make a 7.04 %(w/v) aqueous solution in a 50.0 mL volumetric flask?
Submit Answer
Retry Entire Group
9 more group attempts remaining
req
2req
2req
= 2req
ts 2req
pts 2req
Email In
Cengage Learning | Cengage Technical Support
国
98°F Rain coming
PrtScn
PgDn
F12
Home
End
F10
Pgup
F3
F4
FS
F6
F7
F8
F9
&
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning