Rh Pd Ag Cd In Sn Sb Te | Xe Ir Pt Au Hg TI Pb Bi Po At Rn Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Pu Am Cm Bk Cr Es Fm Md No Lr ted to form| ] covalent bond(s) in order to obey the octet rule. ula of the compound that would form between carbon and chlorine , if the molecule contains only one carbon atom and only single bonds are
Rh Pd Ag Cd In Sn Sb Te | Xe Ir Pt Au Hg TI Pb Bi Po At Rn Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Pu Am Cm Bk Cr Es Fm Md No Lr ted to form| ] covalent bond(s) in order to obey the octet rule. ula of the compound that would form between carbon and chlorine , if the molecule contains only one carbon atom and only single bonds are
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 171CP: Consider the following data for three binary compounds of hydrogen and nitrogen: %H (by Mass) %N...
Related questions
Question
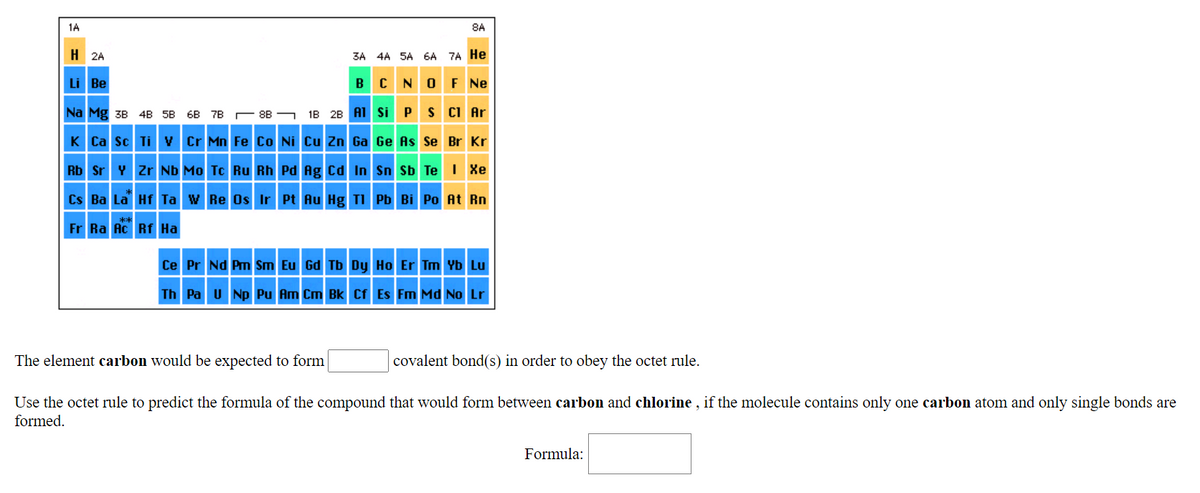
Transcribed Image Text:1A
8A
H 2A
3A 4A 5A 6A 7A He
Li Be
BC NO F Ne
Na Mg 3B 4B 5B 6B 7B
88 - 18 2B A1 si P S CI Ar
K Ca sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
Cs Ba La Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn
Fr Ra Ac Rf Ha
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
The element carbon would be expected to form
covalent bond(s) in order to obey the octet rule.
Use the octet rule to predict the formula of the compound that would form between carbon and chlorine , if the molecule contains only one carbon atom and only single bonds are
formed.
Formula:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning