Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.101QE: In the 1880s, Frederick Trouton noted that the enthalpy of vaporization of 1 mol pure liquid is...
Related questions
Question

Transcribed Image Text:Reaction B
NAHCO3(s)
Na20(s)
_CO2(g) +
H2O(g)
Run 1: Initial grams of NaHCO3
Theoretical yield (grams) of NażO:
Percent yield (Run 1) =
Run 2: Initial grams of NaHCO3 =
Theoretical yield (grams) of Na2O:
Percent yield (Run 2) =
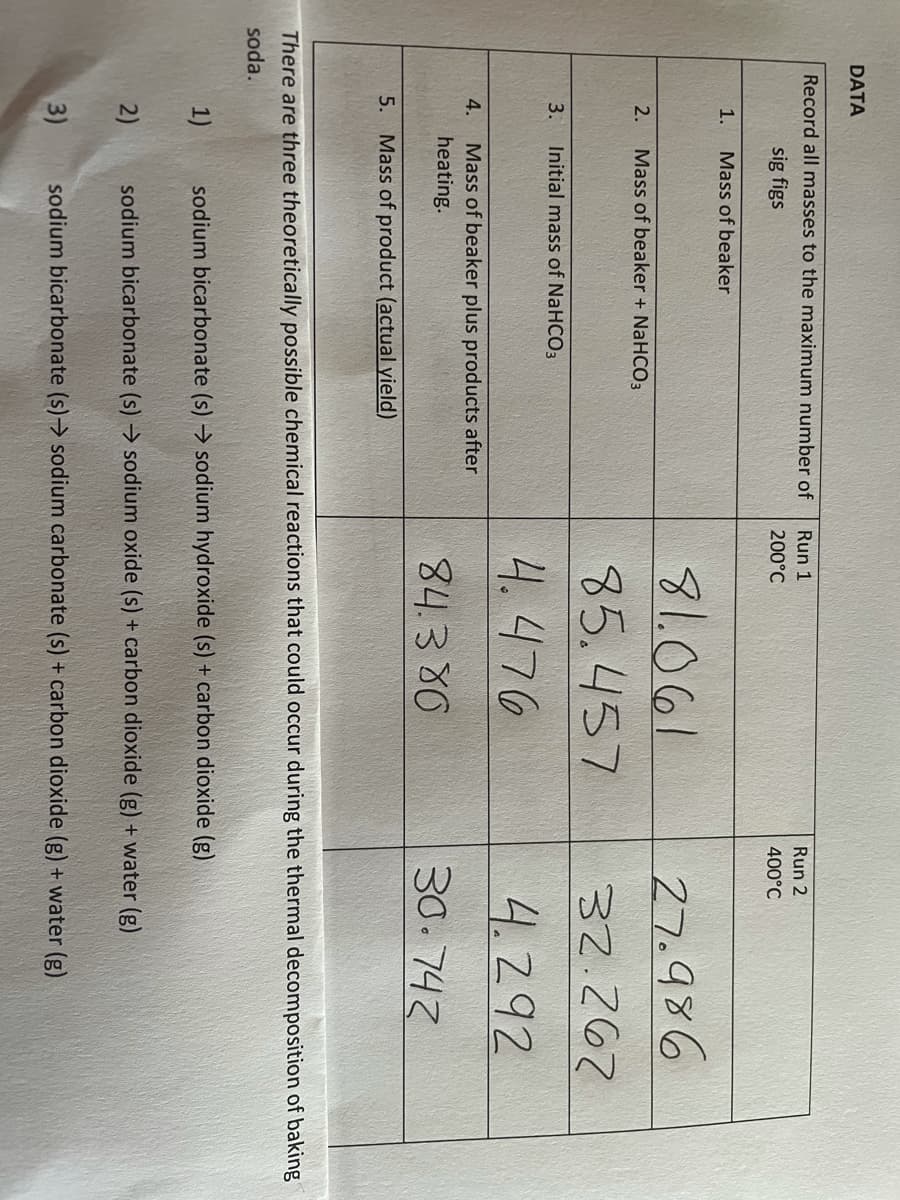
Transcribed Image Text:DATA
Record all masses to the maximum number of
Run 1
Run 2
sig figs
200°C
400°C
1.
Mass of beaker
81.061
27.986
2.
Mass of beaker + NaHCO3
85.457
32.267
3.
Initial mass of NaHCO3
4.476
4.292
Mass of beaker plus products after
heating.
4.
84.3 80
30.742
5.
Mass of product (actual yield)
There are three theoretically possible chemical reactions that could occur during the thermal decomposition of baking
soda.
1)
sodium bicarbonate (s) → sodium hydroxide (s) + carbon dioxide (g)
2)
sodium bicarbonate (s) → sodium oxide (s) + carbon dioxide (g) + water (g)
3)
sodium bicarbonate (s)→ sodium carbonate (s) + carbon dioxide (g) + water (g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning