[S203 2-], M 0.08000 8.0*10-4 0.04000 8.0*10-4 0.02000 8.0*10-4 0.04000 8.0*10-4 0.04000 8.0*10-4 [S208 2-], M Reaction time,s Rate, M/s 49.10 Runs [H, M 1 0.02000 2a 0.02000 106.10 3 0.02000 197.44 4 0.03000 69.20 5 0.04000 53.05 In rate vs In [l-] at constant [S208 2-] In [l-] Runs [l-], M Reaction time, s Rate, M/s In rate 2.00000 4.00000 5.00000 Slope Intercept r2 value Rate order Rounded off Rate orders are typically whole numbers or fractions
[S203 2-], M 0.08000 8.0*10-4 0.04000 8.0*10-4 0.02000 8.0*10-4 0.04000 8.0*10-4 0.04000 8.0*10-4 [S208 2-], M Reaction time,s Rate, M/s 49.10 Runs [H, M 1 0.02000 2a 0.02000 106.10 3 0.02000 197.44 4 0.03000 69.20 5 0.04000 53.05 In rate vs In [l-] at constant [S208 2-] In [l-] Runs [l-], M Reaction time, s Rate, M/s In rate 2.00000 4.00000 5.00000 Slope Intercept r2 value Rate order Rounded off Rate orders are typically whole numbers or fractions
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter10: Energy
Section: Chapter Questions
Problem 10ALQ
Related questions
Question
pls complete the table below using the given data
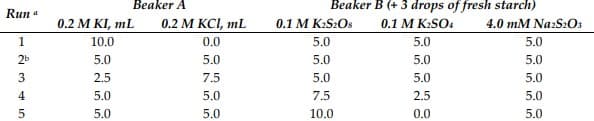
Transcribed Image Text:Beaker A
Beaker B (+ 3 drops of fresh starch)
Run a
0.2 M KI, mL
0.2 М KСL, mL
0.1 M K:S2OS
0.1 M K:SO4
4.0 mM Na:S2O3
1
10.0
0.0
5.0
5.0
5.0
2b
5.0
5.0
5.0
5.0
5.0
2.5
7.5
5.0
5.0
5.0
4
5.0
5.0
7.5
2.5
5.0
5.0
5.0
10.0
0.0
5.0
เก่ เก่ เก่
![|[S203 2-], M
0.08000 8.0*10-4
0.04000 8.0*10-4
0.02000 8.0*10-4
0.04000 8.0*10-4
0.04000 8.0*10-4
Runs
[S208 2-], M
[[H, M
Reaction time,s Rate, M/s
0.02000
49.10
2a
0.02000
106.10
3
0.02000
197.44
4
0.03000
69.20
0.04000
53.05
In rate vs In [l-] at constant [S208 2-]
In [l-]
Runs
[l-], M
Reaction time, s
Rate, M/s
In rate
2.00000
4.00000
5.00000
Slope
Intercept|
r2 value
Rate order
Rounded off
Rate orders are typically whole numbers or fractions](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F55815ef3-1f3f-422e-b33b-6261d3029e90%2Fdba490d5-6dc0-4c18-b4b4-ad0f1831ab37%2Flgzu74_processed.jpeg&w=3840&q=75)
Transcribed Image Text:|[S203 2-], M
0.08000 8.0*10-4
0.04000 8.0*10-4
0.02000 8.0*10-4
0.04000 8.0*10-4
0.04000 8.0*10-4
Runs
[S208 2-], M
[[H, M
Reaction time,s Rate, M/s
0.02000
49.10
2a
0.02000
106.10
3
0.02000
197.44
4
0.03000
69.20
0.04000
53.05
In rate vs In [l-] at constant [S208 2-]
In [l-]
Runs
[l-], M
Reaction time, s
Rate, M/s
In rate
2.00000
4.00000
5.00000
Slope
Intercept|
r2 value
Rate order
Rounded off
Rate orders are typically whole numbers or fractions
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning