se ( x Edward V x O procedur x G traductor x E Imt rgt c x Course H X Part The x Part A A X course.html?courseld=17244391&0penVellumHMAC=Dad27de605f424ee63856c9b0420083fd#10001 Part C The balanced reaction for the combustion of ethane is shown in the table. You allow 2.4 mol of ethane (C;Hs) to react with 3.5 mol oxygen (O2). Complete the following ICF table, which may be done using the Simulation, to indicate the amounts of all chemical species for the initial, change, and final conditions. Drag the appropriate amounts to their respective targets. > View Available Hint(s) Reset Help 14 mol 7.2 mol 00mol 8.4 mol 48 mol 10 mol 24 mol 20 mol 30 mol 3.5 mol 2C;H(g) + 702(g) → 4CO2(g) + 6H20(g) Initial Change Final P Pearson Copyright © 2022 Pearson Education Inc. All rights reserved. | Terms of Use I Privacy Policy I Permissions | Contact Us|
se ( x Edward V x O procedur x G traductor x E Imt rgt c x Course H X Part The x Part A A X course.html?courseld=17244391&0penVellumHMAC=Dad27de605f424ee63856c9b0420083fd#10001 Part C The balanced reaction for the combustion of ethane is shown in the table. You allow 2.4 mol of ethane (C;Hs) to react with 3.5 mol oxygen (O2). Complete the following ICF table, which may be done using the Simulation, to indicate the amounts of all chemical species for the initial, change, and final conditions. Drag the appropriate amounts to their respective targets. > View Available Hint(s) Reset Help 14 mol 7.2 mol 00mol 8.4 mol 48 mol 10 mol 24 mol 20 mol 30 mol 3.5 mol 2C;H(g) + 702(g) → 4CO2(g) + 6H20(g) Initial Change Final P Pearson Copyright © 2022 Pearson Education Inc. All rights reserved. | Terms of Use I Privacy Policy I Permissions | Contact Us|
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 9RQ: What characterizes an electrolytic cell? What is an ampere? When the current applied to an...
Related questions
Question
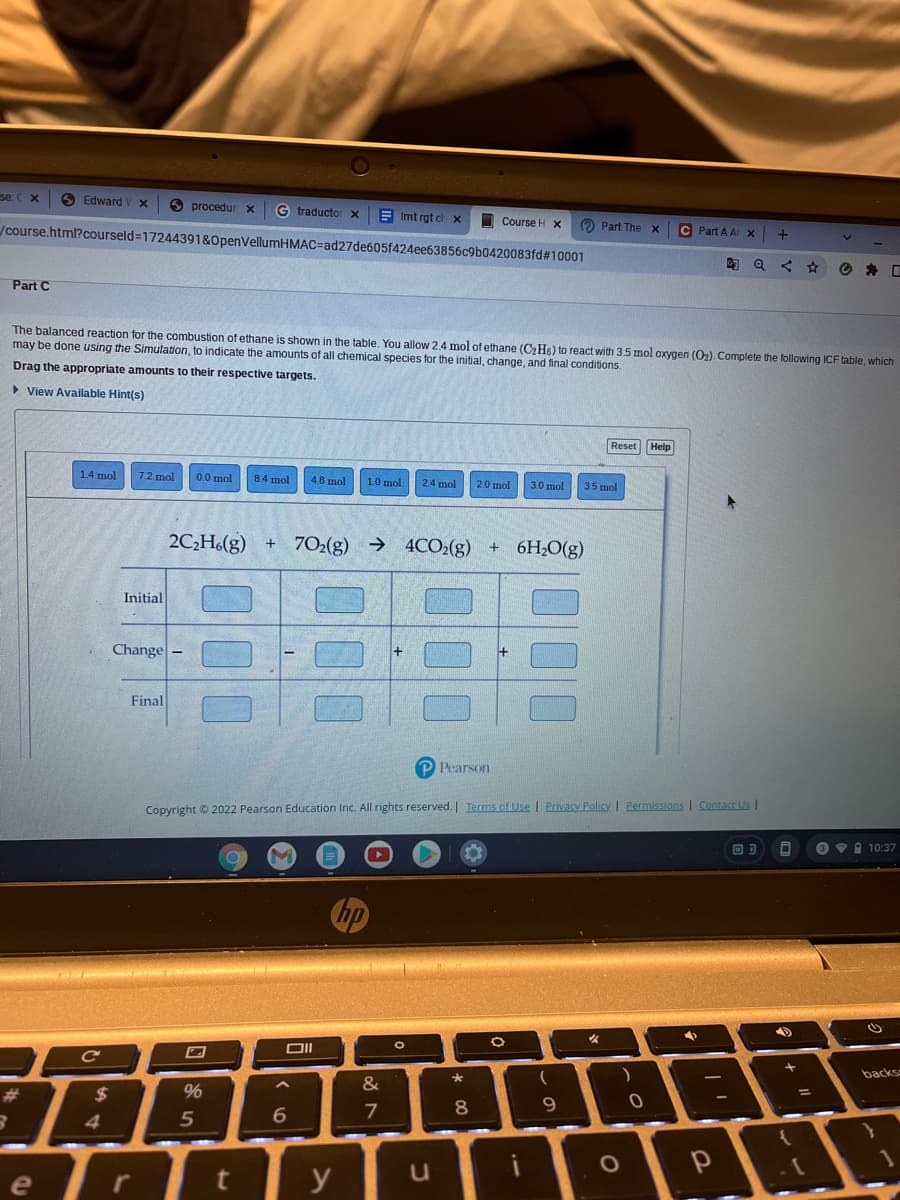
Transcribed Image Text:se: C x
O
Edward V x
O
procedur x
G traductor x
E
Imt rgt ch x
O Course H X
O Part The x
C Part A A x
/course.html?courseld=17244391&0penVellumHMAC=Dad27de605f424ee63856c9b0420083fd#10001
Part C
The balanced reaction for the combustion of ethane is shown in the table, You allow 2,4 mol of ethane (C2H6) to react with 3.5 mol oxygen (O2). Complete the following ICF table, which
may be done using the Simulation, to indicate the amounts of all chemical species for the initial, change, and final conditions.
Drag the appropriate amounts to their respective targets.
> View Available Hint(s)
Reset Help
14 mol
7.2 mol
0.0 mol
8.4 mol
4.8 mol
1.0 mol
24 mol
2.0 mol
3.0 mol
3.5 mol
2C,H(g) + 7O2(g) → 4CO2(g) + 6H20(g)
Initial
Change
Final
P Pearson
Copyright © 2022 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy I Permissions I Contact Us |
O VI 10:37
DII
backs
&
%23
%$4
%
7
4.
5
u
r
t
y
e
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning