Select the best possible products for the given reaction. Don't worry about balancing. Na₂O + H₂O - Bisn Na Mg 2340 Be Fr Rb Sr kad rea NaOH 21 32 Ca Sc Ti Ba Ra 18 Series Na(OH)2 NaH₂O NaO + H₂ Na₂O + H₂ NB 48 5 58 G VIB 65 15-103 104 105 106 Rf befedron Periodic Table of the Elements Zr Nb Mo Tc 7 VIB Ma 5 Abo Hf Ta W Re Os Ir H Engin BUT Cuttors Tato Metal Symbol 24 25 26 27 Cr Mn Fe Co Ni Cu Zn Ha Back 10 12 4345 Ru Rh Pd Ag Cd Bebe Fin T 20 Mela 13 IBA 34 14 IVA Pt Au Hg Tl Al Si Ga Ge As 15 VA BOWTE 16 VIA 64 105 113 112 113 114 115 117 115 c Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb The 35036 LINKKI MER Ac Th Pa Lentionide Se Sn Sb Te Pb Bi Po At Rn Pa Br T 17 VIA 7A F Ne Ar Np Pu Am Cm Bk Cf Es Fm Md No Awekim Derin VIA RA He Kr 24TH SPRAAT 24 GAD I Xe Lu Lr Lawencias
Select the best possible products for the given reaction. Don't worry about balancing. Na₂O + H₂O - Bisn Na Mg 2340 Be Fr Rb Sr kad rea NaOH 21 32 Ca Sc Ti Ba Ra 18 Series Na(OH)2 NaH₂O NaO + H₂ Na₂O + H₂ NB 48 5 58 G VIB 65 15-103 104 105 106 Rf befedron Periodic Table of the Elements Zr Nb Mo Tc 7 VIB Ma 5 Abo Hf Ta W Re Os Ir H Engin BUT Cuttors Tato Metal Symbol 24 25 26 27 Cr Mn Fe Co Ni Cu Zn Ha Back 10 12 4345 Ru Rh Pd Ag Cd Bebe Fin T 20 Mela 13 IBA 34 14 IVA Pt Au Hg Tl Al Si Ga Ge As 15 VA BOWTE 16 VIA 64 105 113 112 113 114 115 117 115 c Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb The 35036 LINKKI MER Ac Th Pa Lentionide Se Sn Sb Te Pb Bi Po At Rn Pa Br T 17 VIA 7A F Ne Ar Np Pu Am Cm Bk Cf Es Fm Md No Awekim Derin VIA RA He Kr 24TH SPRAAT 24 GAD I Xe Lu Lr Lawencias
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 9RQ: What characterizes an electrolytic cell? What is an ampere? When the current applied to an...
Related questions
Question
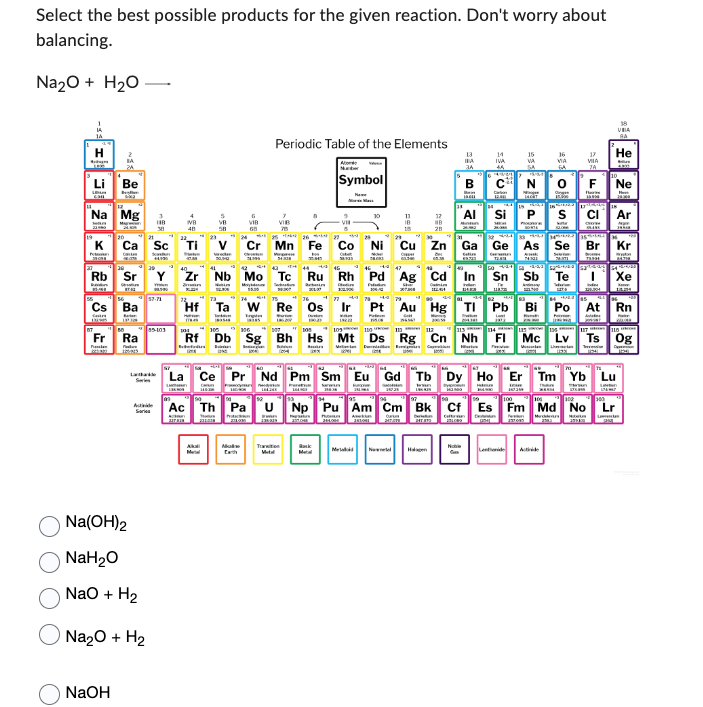
Transcribed Image Text:Select the best possible products for the given reaction. Don't worry about
balancing.
Na₂O + H₂O -
Na Mg
2340
Be
Fr
Rb Sr
kad
rea
NaOH
21
32
Ca Sc Ti
Ba
Ra
18
57.71
15-103
Series
Na(OH)2
NaH₂O
NaO + H₂
Na₂O + H₂
NB
48
5
58
G
VIB
65
Periodic Table of the Elements
7
VIB
Zr Nb Mo Tc
Ma
5
Abo
Tato
Metal
Symbol
Hf Ta W Re Os Ir
H
Engin
BUT
Cuttors
24
25 26 27
Cr Mn Fe Co Ni Cu Zn
Ha
Back
10
12
4345
Ru Rh Pd Ag Cd
Bebis
Fin
20
Mela
13
IBA
34
Pt Au Hg Tl
Np Pu Am Cm Bk Cf
Awekim
Derin
14
IVA
15
VA
Al Si
3458862 356884
Ga Ge As Se Br
T
16
VIA
64
BOWTE
105
113 112
113 114 115
117 115 c
104 105 106
Rf
befedron
Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
The
35036
LINKKI
HOM
MER
Ac Th Pa
Lentionide
17
VIA
7A
F Ne
Ar
VIA
RA
He
Kr
24TH
STRAAT 24 LED
Xe
Sn Sb Te
Pb Bi Po At Rn
Es Fm Md No
Lu
Lr
Lawencias
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning