Select the set of quantum numbers that represents each electron in a ground-state Be atom. n = 1, l = 0, mẹ = -1, m, =+; %3D %3D n = 2, l = 1, mẹ = 0, m, = %3D – 2 Un = 2, l = 1, mẹ = 1, m, = + n = 2, l = 0, mę = 0, ms = - п %3 2, 0 %3D 0, т, 3D 0, т, — —1 = -1 n = 2, l = 0, mẹ = 0, m, = +} n = 1, l = 0, mę = 0, m, = - On = 1, l = 0, mẹ = 0, m, = +
Select the set of quantum numbers that represents each electron in a ground-state Be atom. n = 1, l = 0, mẹ = -1, m, =+; %3D %3D n = 2, l = 1, mẹ = 0, m, = %3D – 2 Un = 2, l = 1, mẹ = 1, m, = + n = 2, l = 0, mę = 0, ms = - п %3 2, 0 %3D 0, т, 3D 0, т, — —1 = -1 n = 2, l = 0, mẹ = 0, m, = +} n = 1, l = 0, mę = 0, m, = - On = 1, l = 0, mẹ = 0, m, = +
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter6: The Structure Of Atoms
Section6.6: The Shapes Of Atomic Orbitals
Problem 2RC: Which of the following sets of quantum numbers correctly represents a 4p orbital? (a) n = 4, = 0, m...
Related questions
Question
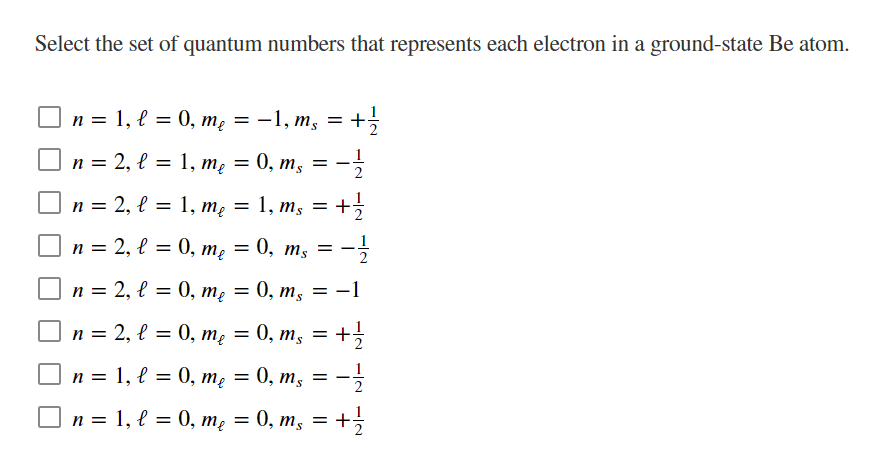
Transcribed Image Text:Select the set of quantum numbers that represents each electron in a ground-state Be atom.
O n = 1, l = 0, mẹ = -1, m, = +
%3D
S.
n = 2, l = 1, mę = 0, m, = -
n = 2, l = 1, mę = 1, m, = +
}
O n = 2, l = 0, mẹ = 0, m, =
2
n = 2, l = 0, mę
0, т, 3D —1
п 3 2, l 3D0, т, — 0, т, — +
S.
O n = 1, l = 0, mẹ = 0, m, = -
1
2
п %3D 1, е %3D 0, т, 3D 0, т, — +>3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning