Set up the problem and calculate the mass of 18.7 mL of nickel. See the table below for the density of nickel. Densities of Some Common Substances Substance Density (g/mL) Water 1.00 Aluminum 2.72 Chromium 7.25 8.91 8.94 10.50 Nickel Copper Silver Lead Mercury Gold Tungsten Platinum 18.7 mL Ni x 11.34 13.60 19.28 19.38 21.46 || g Ni
Set up the problem and calculate the mass of 18.7 mL of nickel. See the table below for the density of nickel. Densities of Some Common Substances Substance Density (g/mL) Water 1.00 Aluminum 2.72 Chromium 7.25 8.91 8.94 10.50 Nickel Copper Silver Lead Mercury Gold Tungsten Platinum 18.7 mL Ni x 11.34 13.60 19.28 19.38 21.46 || g Ni
Mathematics For Machine Technology
8th Edition
ISBN:9781337798310
Author:Peterson, John.
Publisher:Peterson, John.
Chapter63: Volumes Of Pyramids And Cones
Section: Chapter Questions
Problem 8A
Related questions
Question
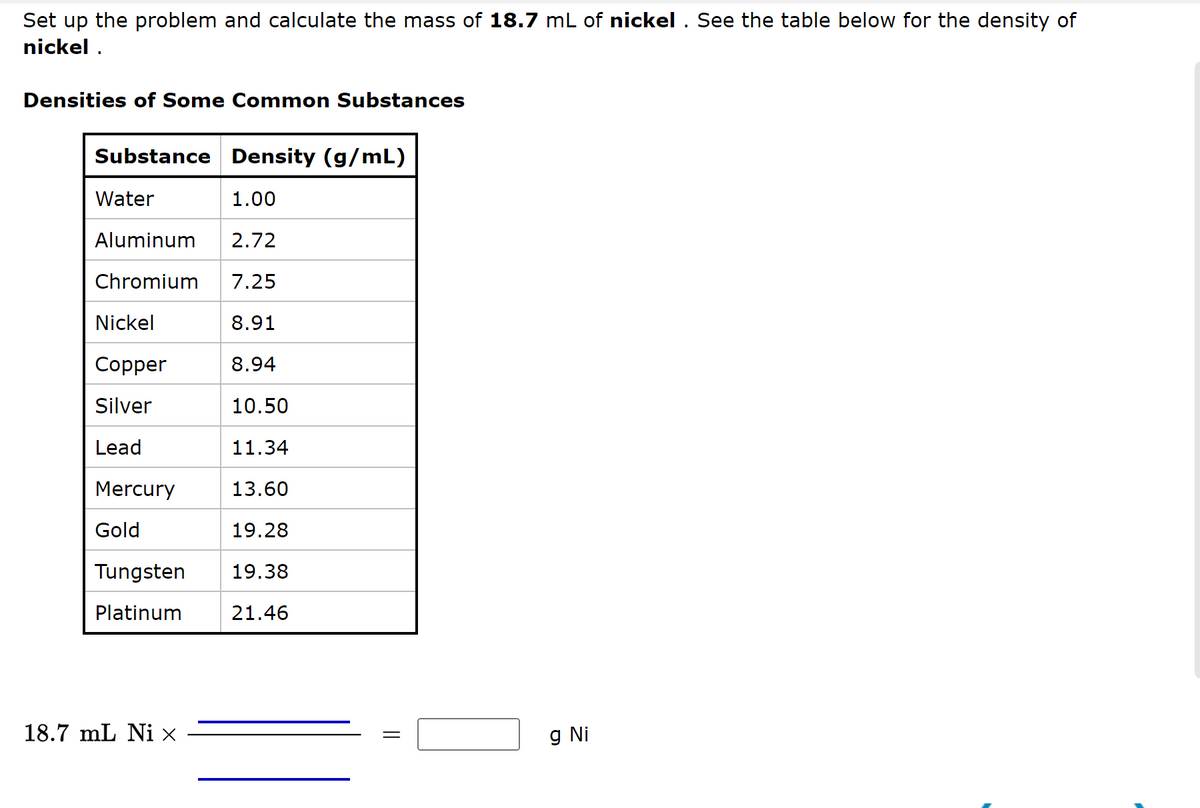
Transcribed Image Text:Set up the problem and calculate the mass of 18.7 mL of nickel. See the table below for the density of
nickel.
Densities of Some Common Substances
Substance Density (g/mL)
Water
1.00
Aluminum 2.72
Chromium 7.25
8.91
8.94
10.50
11.34
13.60
19.28
19.38
21.46
Nickel
Copper
Silver
Lead
Mercury
Gold
Tungsten
Platinum
18.7 mL Ni x
||
g Ni
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Mathematics For Machine Technology
Advanced Math
ISBN:
9781337798310
Author:
Peterson, John.
Publisher:
Cengage Learning,

Elementary Geometry For College Students, 7e
Geometry
ISBN:
9781337614085
Author:
Alexander, Daniel C.; Koeberlein, Geralyn M.
Publisher:
Cengage,

Algebra: Structure And Method, Book 1
Algebra
ISBN:
9780395977224
Author:
Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:
McDougal Littell

Mathematics For Machine Technology
Advanced Math
ISBN:
9781337798310
Author:
Peterson, John.
Publisher:
Cengage Learning,

Elementary Geometry For College Students, 7e
Geometry
ISBN:
9781337614085
Author:
Alexander, Daniel C.; Koeberlein, Geralyn M.
Publisher:
Cengage,

Algebra: Structure And Method, Book 1
Algebra
ISBN:
9780395977224
Author:
Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:
McDougal Littell