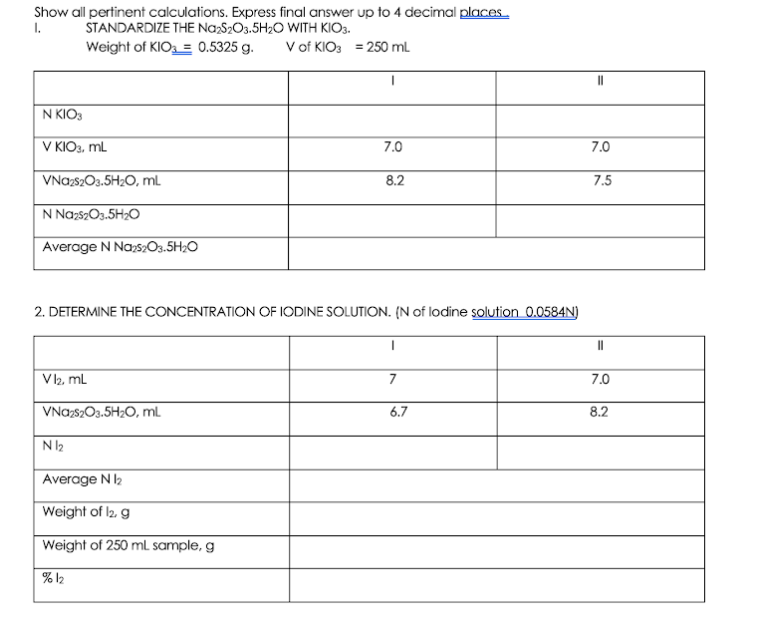
Show all pertinent calculations. Express final answer up to 4 decimal places I. STANDARDIZE THE NazS2O3.5H;O WITH KIO3. Weight of KIO = 0.5325 g. V of KIO3 = 250 mL
Q: A 40.00 ml sample of 0.1000 M diprotic malonic acid is titrated with 0.0900 M KOH. What volume KOH…
A:
Q: how many milliliters of 2.00 M h2so4 will react with 28.0g of sodium hydroxide?
A:
Q: 21) Pease qui the appropuate named on genal kypes g veastuins heeded to Complute the Syntess. 2? 3?
A: Since, this is a multiple questions. So, I will solved first question ( 21 ) for you with details…
Q: Classification: 2) What is the heat change when 1.48 g of Chlorine reacts with excess phosphorus…
A:
Q: Chemistry According to the following reaction, how many grams of mercury(II) oxide are necessary to…
A: Mole can be defined as the standard unit for measuring large quantities of very small entities such…
Q: 1. If the Ka of an acid is 1.8x10 ^-5, calculate the pKa and pKb values of the acid.
A: Since you have asked multiple type questions, we will solve only first questions for you. If you…
Q: LANCE THE EQUATION FIRST) 1. Compute for the ΔG of the equation: ___________kJ/mol (Write your…
A: So ∆Hf = -40.05 KJ/mol
Q: A mixture of species of A, B, and C is injected into a liquid chromatographic column with length of…
A: Given,Length of the column = 20.0 cmSpecies Retention time min…
Q: solution was prepared by mixing 100.0 mL of 4.0 x 10-2 M Fe(NO3); and 100.0 mL of 2.0 × 10-4 M NAOH.…
A:
Q: Draw a ketohexose. Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and…
A: We have to draw a ketohexose.
Q: 4. Complete the multistep syntheses below. OEt
A: In this question, we will synthesized the final products by using all the reagents, and this is a…
Q: Consider a buffer made by adding 43.1 g of (CH:):NH21 to 250.0 mL of 1.42 M (CH.):NH (Kb = 5.4 x…
A:
Q: 94. Balance these neutralization reactions: a. HNO, + Ba(OH), → Ba(NO3)2 + H,O b. HF + Ba(OH),→ BaF,…
A:
Q: 64. The following molecular equations each show an ionic compound dissolving in water. Show the…
A:
Q: (8) Discuss the three main processes of interactions of electrons with matter.
A: Charged particles interact with matter via elastic and inelastic collisions with atomic electrons…
Q: The boiling point of ethanol, CH3CH2OH, is 78.500 °C at 1 atmosphere. Kp(ethanol) = 1.22 °C/m In a…
A:
Q: Properties of soap and detergent SOAP DETERGENT Emulsifying ability
A:
Q: Exercise 6 Ethane burns in air to give carbon dioxide and water according to the following…
A:
Q: Question 25 Which of the following amino acids is a secondary amine? A. proline B. glutamine C.…
A: The given problem related to the aminoacids topic.
Q: Explain per step. Given the MS spectra of 2-pentanol, show the fragmentation pattern responsible…
A: The mass spectrum of 2-pentanol given has peaks at m/z 73, 70, 55, 45, 42.
Q: Draw the structure of the compound whose data is shown below, then select all functional groups in…
A: The IR helps us to find the possible functional group may present in the unknown organic molecule.…
Q: A. Determination of Solubility and Ksp 1. Consider 50 mL of a saturated solution of PbC12 at 250C.…
A: Given : Volume of saturated solution = 50 ml Mass of solid PbCl2 obtained = 0.54 g
Q: exothermic reaction?
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Determine if the acid-base neutralization reaction of hydrochloric acid (HCI) with sodium hydroxide…
A:
Q: H. 1. NaNH2 (3 equiv) Br2 2. H20 OH Na NH3 (1) OH
A:
Q: time [H,co,] (seconds) 0.800M 1.0 0.384 M 2.0 0.185 M 3.0 0.0888 M 4.0 0.0426M
A: rate law states that rate of reaction is directly propertional to the product of molar concentration…
Q: What buffer system should you use if the desired pH is 6? desired pH is 9? 2. When a strong acid…
A: 1. Citrate buffer can be used if the desired ph is 6. Preparation : 1.prepare 800 mL of distilled…
Q: for 2Al(OH)3.. what is the 2 called and why is it there? what is 3 called and why is it there?…
A: Balanced chemical equation is equation in which number of atoms on both sides of equation are…
Q: (a)Calculate the radius (in m) of the orbit for the innermost electron in osmium assuming it is…
A: know that Bhores radius is given as Rn = n2 r0 / Z Here n = Principle quatum number r0 = Ground…
Q: out acid base equilibria and explain to me step by step on whats happening in the video with long…
A: Acid base equilibria: Reagents and tools used are, 0.1 M HCl 0.1 M acetic acid Distilled water…
Q: Complete and Balance The following equation, in the case that the reaction doesnt occur write "NR"…
A: Given unbalanced and incomplete reaction is : MnO4- + OH- + SO32- --------------> ? Complete…
Q: Chemistry Chemistry Steps NO2 Br2 а) FeBr3 CH3 Chemistry Chemistry Steps Cl2 b) ? AICI3 СООН…
A:
Q: Propose a mechanism for each of the following reaction: CH3 H20 Br но CH3
A: In this reaction mechanism step first formation of carbocation step second in the ring opening and…
Q: Question 3 What variable is correlated in the titration based on Nernst equation? Potential energy…
A: Nernst Equation Ecell = potential energy of the cell E°cell = standard potential energy of the…
Q: 4. State whether these two are: CH, CH, a) the same molecule b) different compounds Cl- Br Br -Cl…
A: Detail description is given below . After r/s designation if both give same configuration then they…
Q: A solution prepared by dissolving 25.8 mg of benzene (78.11 g/mol) in hexane (86.16 g/mol) and…
A: Here we have to just calculate the concentration. We don't require the absorption value. The…
Q: Question 5 What is the electrophile in the reaction of benzene with acetyl chloride, CH;COCI, and…
A: The electrophile in reaction of benzene with acetyl chloride, and Aluminium Chloride is acetyl…
Q: A mixture of species of A, B, and C is injected into a liquid chromatographic column with length of…
A: Chromatography is an analytical and separation technique which is used to separate the components…
Q: 2. Increasing in temperature: a. H20a) = H20) (+AH) b. N2(9) + 3H2(9) 2 2NH3(9) (-AH); c. If the…
A:
Q: Theme: Limiting and Excess Reactants Fe2O3 + 2Al → Al2O3 + 2Fe If I started with 25 moles of Fe2O3…
A: Given, moles of Fe2O3 = 25 mol moles of Al = 43 mol The balamced chemical reaction is Fe2O3 + 2 Al…
Q: 13.7 mL of CO2 is held at 11.7atm and 20°C in a fire extinguisher. When the extinguisher is used,…
A: Here pressure, volume and temperature all are changing to combined gas law would be used to…
Q: systematic
A: ✓An interferent is a species that cause an error in an analysis by enhancing or attenuating…
Q: 3. Which do itself? Why? guhic Chemistry an aromatic ion precursor? 9-bromocyclononatetraene or…
A: The reactants given are 9-bromocyclononatetraene and cyclononatetraene.
Q: Draw and identify (R) and (S) enantiomers of lactic acid, CH3CH(OH)COOH. Use the proper conventions…
A:
Q: 4. Is the acid-base neutralization of HCl and NaOH spontaneous or non-spontaneous at 37°C? (Write…
A:
Q: Given the following values, what is the concentration of the acid? (With solution) Initial Volume…
A: Given: Initial volume of acid = 10.150 mL Final volume of acid = 11.620 mL Initial volume of NaOH =…
Q: A rock formed with 1000 atoms of a radioactive parent element, but only contains 250 radioactive…
A: Given, A rock formed with 1000 atoms of a radioactive parent element, but only contains 250…
Q: Which is the structure of the substance which is important in the detoxification mechanism of…
A:
Q: A coffee-cup calorimeter contains 70 mL of water with an initial temperature of 24.9°C. An unknown…
A:
Q: Which of the following is not used as a primary standard to standardize acidic titrants? Section а.…
A: Primary standard are those compounds which are sufficiently pure such that their solution can be…
answer 1
Step by step
Solved in 6 steps with 1 images

- Express 20,000ppb as a percentage strength A. 2% B. 0.02% C. 0.00002% D. 0.002%Design a procedure on how you will prepare 1.25 M table sugar (C12H22O11)solution and 1.25 M table salt (NaCl) solution diluted to 1 L water. Perform three trialseach set-up and get the average. Write/ encode your output in short bond paper withthe following parts:Title____________________I. Objectives:II. Materials:III. Procedure (including pictures/diagrams):IV. Data:V. Generalization: (Note: Perform this activity on a separate table/ area at home to ensure accuracyand precision of data. Be careful on handling the solution to avoid spillage.)1. Calculate the experimental density of a salt solution and the percent error (same as relative error percent) using some or all the data given below. solubility of NaCl salt in water: 0.357 g/mLmass of empty graduated cylinder: 25.19g mass of graduated cylinder + salt solution: 30.47g total volume of salt solution: 4.98 mLtrue density of salt solution: 1.07 g/mL
- Hello can this problem be simplified In administering a mixture of NO2 and O2 by flow meters the target total flow is 14 L/min. What flow , in L/min should the NO2 have to administer 31 % NO2 ? (answer to 1/10 X.X and include units) Answer unitsPlease do not round off intermediate calculations. Thank you.using exactly 5.00 mL of 0.0400 M stock CuSO4 solution. Add 100 mL of water. Data for Part IMass of empty dish: 32.470 g empty dishVolume of 0.0400 M solution: 5.00 mL CuSO4 solution.Mass of dish and 0.0400 M solution: 37.497 g dish and solutionMass of dish and CuSO4 solid: 32.503 g dish and CuSO4 solidCalculations for Part I1. Calculate the mass of solution2. Calculate the mass of solid CuSO4 dissolved in the solution.3. Calculate the number of moles of solid CuSO4 dissolved in the solution.4. Calculate the mass of water evaporated from the solution.5. Calculate the density of solution, (g solution/mL solution).6. Calculate the % by mass, CuSO4 in solution (100 x g CuSO4/g solution).7. Calculate the molality of solution (moles CuSO4/kg solvent).8. Calculate the molarity of solution (moles CuSO4/L solution).9. Given that the true molarity is 0.0400 M, calculate the percent error of your result.
- using exactly 5.00 mL of 0.0400 M stock CuSO4 solution. Add 100 mL of water. Data for Part IMass of empty dish: 32.470 g empty dishVolume of 0.0400 M solution: 5.00 mL CuSO4 solution.Mass of dish and 0.0400 M solution: 37.497 g dish and solutionMass of dish and CuSO4 solid: 32.503 g dish and CuSO4 solidCalculations for Part I1. Calculate the mass of solution? 5.027 g 2. Calculate the mass of solid CuSO4 dissolved in the solution? 0.033 g 3. Calculate the number of moles of solid CuSO4 dissolved in the solution? 2.07 x 10-4 mol4. Calculate the mass of water evaporated from the solution? 0.0414 M5. Calculate the density of solution, (g solution/mL solution)?6. Calculate the % by mass, CuSO4 in solution (100 x g CuSO4/g solution)?7. Calculate the molality of solution (moles CuSO4/kg solvent)?8. Calculate the molarity of solution (moles CuSO4/L solution)?9. Given that the true molarity is 0.0400 M, calculate the percent error…Please answer 1-2 sub-units thank you

