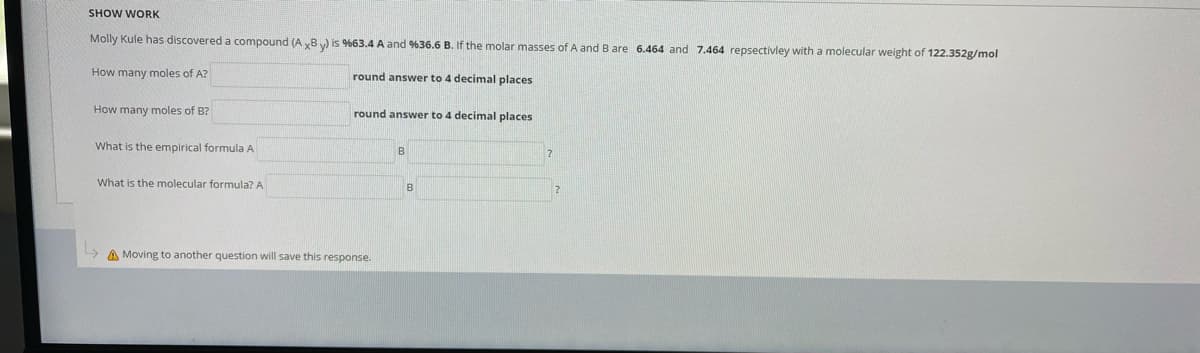
SHOW WORK Molly Kule has discovered a compound (A y8) is %63.4 A and %36.6 B. If the molar masses of A and B are 6.464 and 7.464 repsectivley with a molecular weight of 122.352g/mol How many moles of A? round answer to 4 decimal places How many moles of B? round answer to 4 decimal places What is the empirical formula A What is the molecular formula? A B
SHOW WORK Molly Kule has discovered a compound (A y8) is %63.4 A and %36.6 B. If the molar masses of A and B are 6.464 and 7.464 repsectivley with a molecular weight of 122.352g/mol How many moles of A? round answer to 4 decimal places How many moles of B? round answer to 4 decimal places What is the empirical formula A What is the molecular formula? A B
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 3ALQ: True or false? The atom with the largest subscript in a formula is die atom with the largest percent...
Related questions
Question
Transcribed Image Text:SHOW WORK
Molly Kule has discovered a compound (A yB) is %63.4 A and %36.6 B. If the molar masses of A and B are 6.464 and 7.464 repsectivley with a molecular weight of 122.352g/mol
How many moles of A?
round answer to 4 decimal places
How many moles of B?
round answer to 4 decimal places
What is the empirical formula A
What is the molecular formula? A
B
A Moving to another question will save this response.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.