Shown here is a table of the vapor pressure of Vapor Pressure (kPa) benzene and toluene. Temperature (°C) Benzene Toluene 85 116.9 46.0 Estimate the heat of vaporization of benzene and of toluene at 85 °C and at 105 °C using the highest order of accuracy possible. Heat of vaporization can be calculated using the Clausius-Clapeyron 90 135.5 54.0 95 155.7 63.3 100 179.2 74.3 Relation: 105 204.2 86.0 d In P ΔΗ dT RT2
Shown here is a table of the vapor pressure of Vapor Pressure (kPa) benzene and toluene. Temperature (°C) Benzene Toluene 85 116.9 46.0 Estimate the heat of vaporization of benzene and of toluene at 85 °C and at 105 °C using the highest order of accuracy possible. Heat of vaporization can be calculated using the Clausius-Clapeyron 90 135.5 54.0 95 155.7 63.3 100 179.2 74.3 Relation: 105 204.2 86.0 d In P ΔΗ dT RT2
Functions and Change: A Modeling Approach to College Algebra (MindTap Course List)
6th Edition
ISBN:9781337111348
Author:Bruce Crauder, Benny Evans, Alan Noell
Publisher:Bruce Crauder, Benny Evans, Alan Noell
Chapter1: Functions
Section1.2: Functions Given By Tables
Problem 2TU: Use the table of values you made in part 4 of the example to find the limiting value of the average...
Related questions
Question
Need asap. Solve using Numerical Differentiation
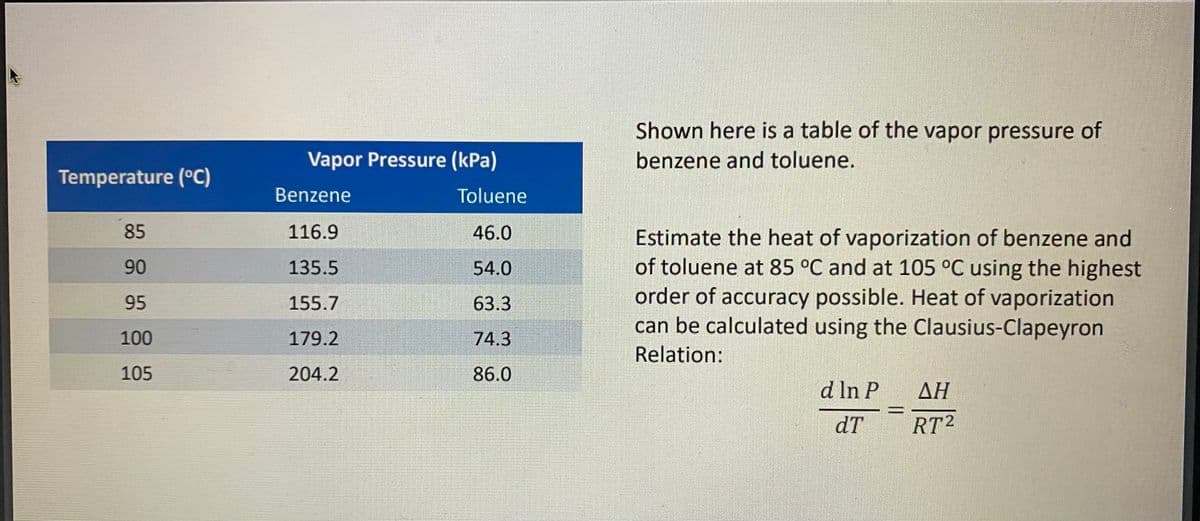
Transcribed Image Text:Shown here is a table of the vapor pressure of
benzene and toluene.
Vapor Pressure (kPa)
Temperature (°C)
Benzene
Toluene
85
116.9
46.0
Estimate the heat of vaporization of benzene and
of toluene at 85 °C and at 105 °C using the highest
order of accuracy possible. Heat of vaporization
can be calculated using the Clausius-Clapeyron
90
135.5
54.0
95
155.7
63.3
100
179.2
74.3
Relation:
105
204.2
86.0
d In P AH
dT
RT2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Functions and Change: A Modeling Approach to Coll…
Algebra
ISBN:
9781337111348
Author:
Bruce Crauder, Benny Evans, Alan Noell
Publisher:
Cengage Learning

Functions and Change: A Modeling Approach to Coll…
Algebra
ISBN:
9781337111348
Author:
Bruce Crauder, Benny Evans, Alan Noell
Publisher:
Cengage Learning