Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.62E
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
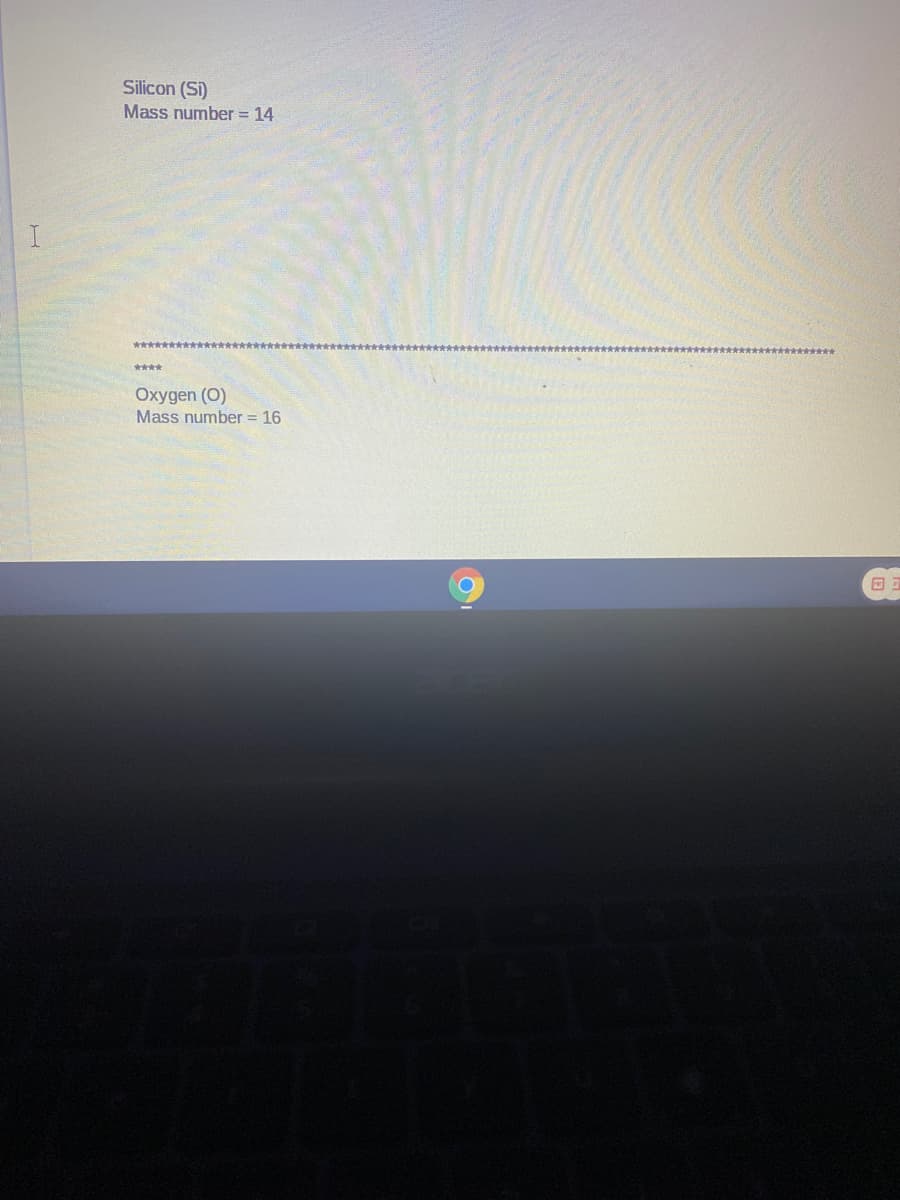
Transcribed Image Text:Silicon (Si)
Mass number = 14
*******
****
Oxygen (O)
Mass number = 16

Transcribed Image Text:Halnium
Tantalum
Platinum
Gold
Morcury
Thalium
Lead
Bamuth
Polonium
Astatine
Tungston
106
dum
Lutotium
Ahenium
Osmium
Iridium
Barlum
88
Ra
103
109
110
111
37
Fr
23)
104
105
107
108
Lr
Rf
Db
Sg
Bh
Hs
Mt
Ds
Rg
Mass numbers in paronthoses are those of
the most stable or most common isotope.
(280)
(270)
Hassium
281)
Meitnerium Damptadum Roantgankum
(268)
(271)
(272)
Bohrlum
(276)
(226)
Radium
(262)
(267)
nclum
Lawrencium uthertordium Dubnium Seabonglum
For each element below, draw a Bohr model that includes the correct numbers for Protons,
neutrons, electrons and energy levels (shells)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER