So the problem is im solving this textbook problem and when im doing the math im getting 271K, what am i doing wrong. I prefer paper responses instead of computer so i can really understand. im horrible at math , plz help. This is the textbook problem: A certain reaction has an activation energy of 54.0 kJ/mol. As the temperature is increased from 22 C to a higher temperature, the rate constant increases by a factor of 7.00. Calculate the higher temperature.
So the problem is im solving this textbook problem and when im doing the math im getting 271K, what am i doing wrong. I prefer paper responses instead of computer so i can really understand. im horrible at math , plz help. This is the textbook problem: A certain reaction has an activation energy of 54.0 kJ/mol. As the temperature is increased from 22 C to a higher temperature, the rate constant increases by a factor of 7.00. Calculate the higher temperature.
Chapter13: Kinetic Methods
Section: Chapter Questions
Problem 8P
Related questions
Question
So the problem is im solving this textbook problem and when im doing the math im getting 271K, what am i doing wrong. I prefer paper responses instead of computer so i can really understand. im horrible at math , plz help. This is the textbook problem: A certain reaction has an activation energy of 54.0 kJ/mol. As the temperature is increased from 22 C to a higher temperature, the rate constant increases by a factor of 7.00. Calculate the higher temperature.
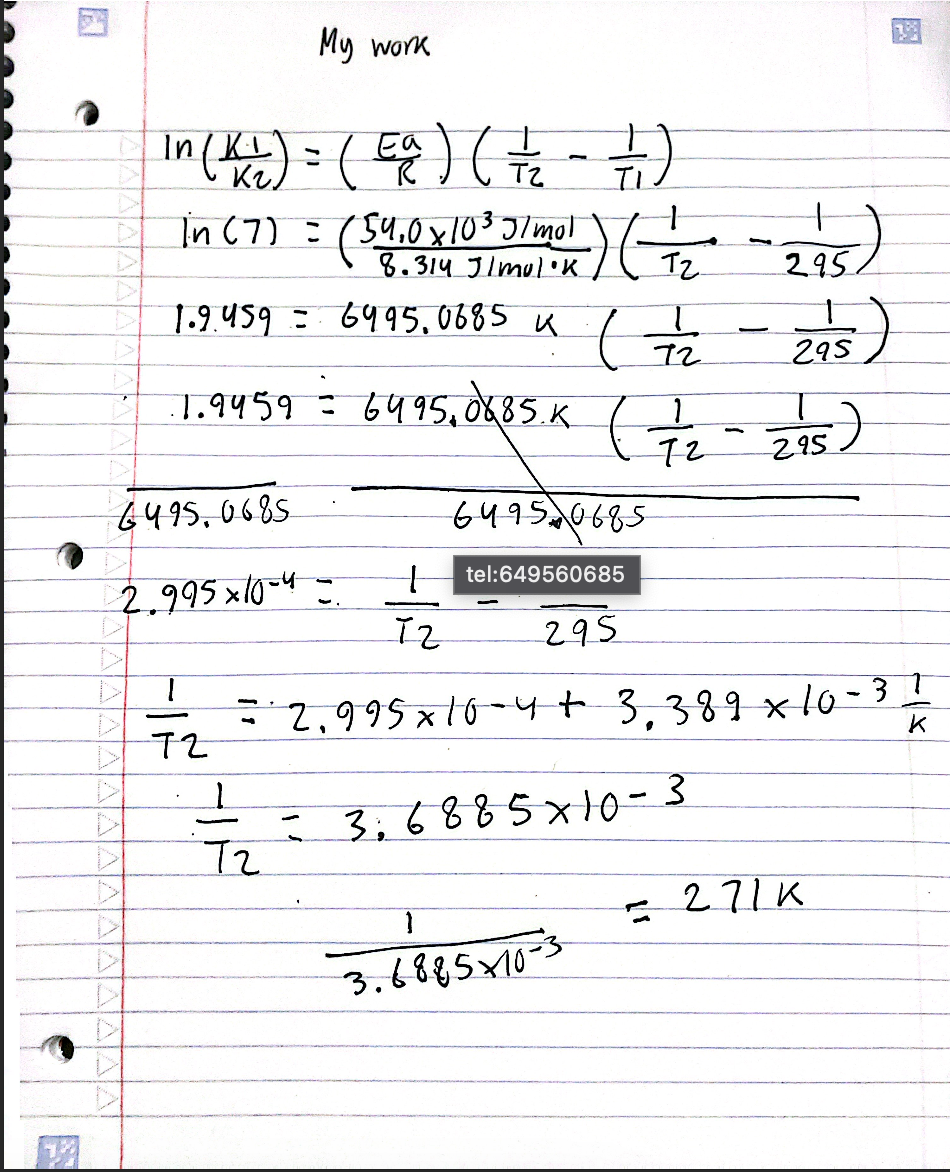
Transcribed Image Text:別
My work
In (K₁) = ( Ea ) ( ½/₂2 - 7 )
T2
In (7) = (540x1013/1) ( 1₂ -295)
T2
1.9.459 = 6995,0685 K ( 72
11.9459 = 6495,0685 K (7/7/₂2
1
6495,0685
2.995×10-4 =
1
72
64950685
+ tel:649560685
IZ
295
1
72
= 3₁6 88 5 x 10-3
-
1
3.6885×10-3
295
= 2.995× 10 -4+ 3,389 x 10-31
к
295
700
= 271K

Transcribed Image Text:#15.
(2) -
In
In(7.00) =
1
12
=
=
Е
1
RT₁ Т2
-
К2
k₁
textbook
=7.00, T1=295 K, Ea = 54.0 x 103 J/mol
5.4 x 104 J/mol
1
8.3145/K . mol 295K
=3.09×10-3, (Tz=324 к=51°C
T2
1
295K
-
1
Т2
=
3.00 × 10-4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 18 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning