Sodium hydroxide is extremely soluble in water. At a certain temperature, a saturated solution contains 569 g NaOH(s) per iter of solution. Calculate the molarity of this saturated NaOH(aq) solution. concentration: 0.6 Incorrect
Sodium hydroxide is extremely soluble in water. At a certain temperature, a saturated solution contains 569 g NaOH(s) per iter of solution. Calculate the molarity of this saturated NaOH(aq) solution. concentration: 0.6 Incorrect
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 151QRT: A mountain lake that is 4.0 km × 6.0 km with an average depth of 75 m has an H+(aq) concentration of...
Related questions
Question
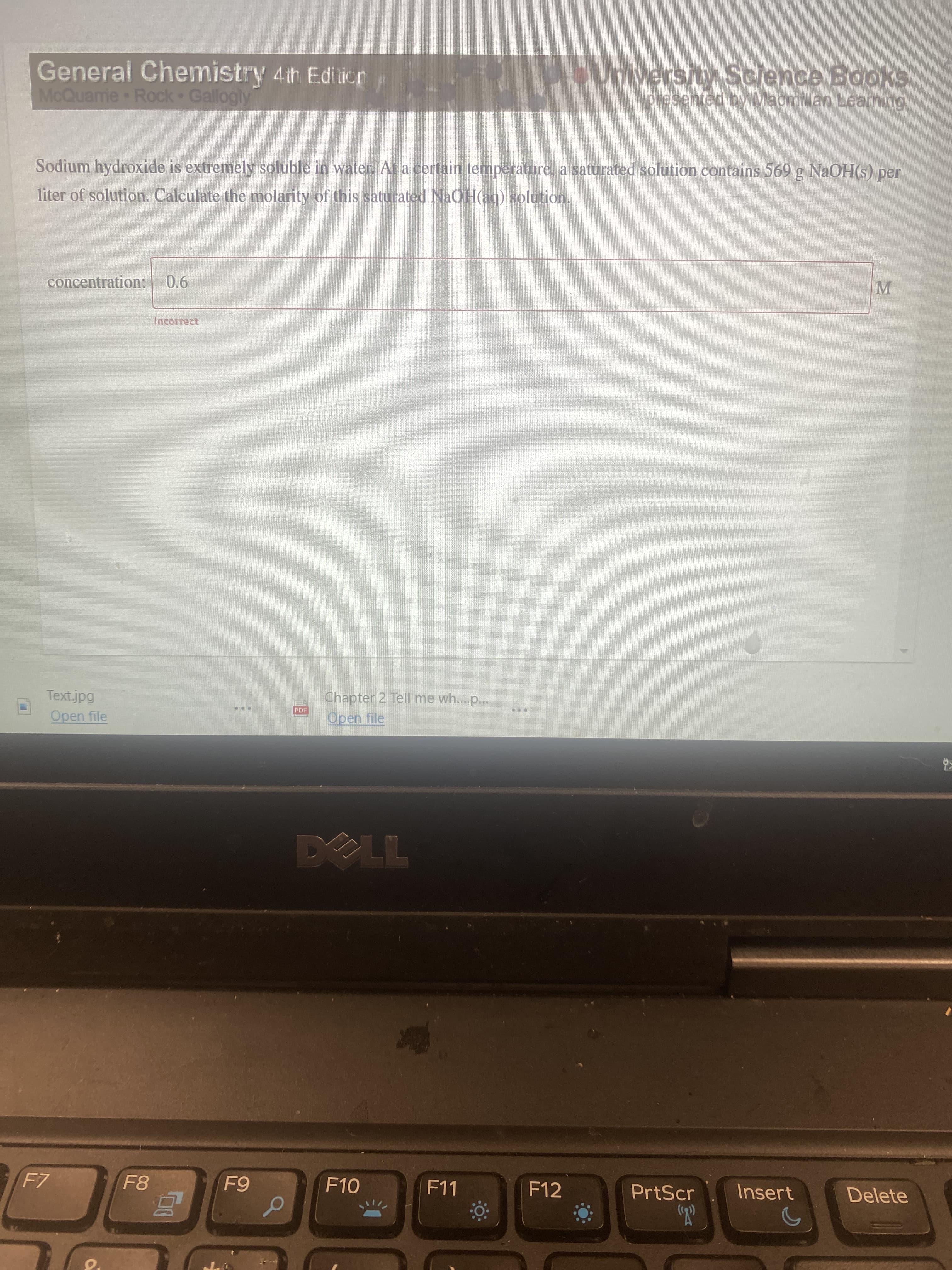
Transcribed Image Text:Sodium hydroxide is extremely soluble in water. At a certain temperature, a saturated solution contains 569 g NaOH(s) per
iter of solution. Calculate the molarity of this saturated NaOH(aq) solution.
concentration:
0.6
Incorrect
Expert Solution
Step 1
Given values are,
Mass of NaOH= 569 g
Volume of solution = 1L
We have formula,
Molarity = moles of solute / litres of solution
Now ,
Molar mass of NaOH= 39.997 g/mol
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER