Solute Carrier (a) (b) (c) Acetone Toluene Ethyl alcohol Ethylene glycol п-Неptane Glycerine Table 8.4 Group Interactions for Solvent Selection Solvent Group Solute 1 4 8. Acid, aromatic OH (phenol) Paraffinic OH (alcohol), water, imide or 0. amide with active H Ketone, aromatic nitrate, tertiary amine, pyridine, sulfone, trialkyl phosphate, or phosphine oxide 4 Ester, aldehyde, carbonate, phosphate, nitrite or nitrate, amide without active H; intramolecular bonding, e.g., o-nitrophenol Ether, oxide, sulfide, sulfoxide, primary and secondary amine or imine Multihaloparaffin with active H Aromatic, halogenated aromatic, olefin 8. Paraffin Monohaloparaffin or olefin 0. (+) Plus sign means that compounds in the column group tend to raise acti vity coefficients of compounds in the row group. (-) Minus sign means a lowering of activity coefficients. (0) Zero means no effect. Choose a solvent that lowers the activity coefficient. 6. | + + + + + + + • + + +
Solute Carrier (a) (b) (c) Acetone Toluene Ethyl alcohol Ethylene glycol п-Неptane Glycerine Table 8.4 Group Interactions for Solvent Selection Solvent Group Solute 1 4 8. Acid, aromatic OH (phenol) Paraffinic OH (alcohol), water, imide or 0. amide with active H Ketone, aromatic nitrate, tertiary amine, pyridine, sulfone, trialkyl phosphate, or phosphine oxide 4 Ester, aldehyde, carbonate, phosphate, nitrite or nitrate, amide without active H; intramolecular bonding, e.g., o-nitrophenol Ether, oxide, sulfide, sulfoxide, primary and secondary amine or imine Multihaloparaffin with active H Aromatic, halogenated aromatic, olefin 8. Paraffin Monohaloparaffin or olefin 0. (+) Plus sign means that compounds in the column group tend to raise acti vity coefficients of compounds in the row group. (-) Minus sign means a lowering of activity coefficients. (0) Zero means no effect. Choose a solvent that lowers the activity coefficient. 6. | + + + + + + + • + + +
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Using Table , select liquid–liquid extraction solvents for removing the solute from the carrier in the following cases:
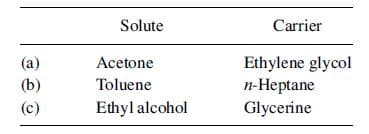
Transcribed Image Text:Solute
Carrier
(a)
(b)
(c)
Acetone
Toluene
Ethyl alcohol
Ethylene glycol
п-Неptane
Glycerine
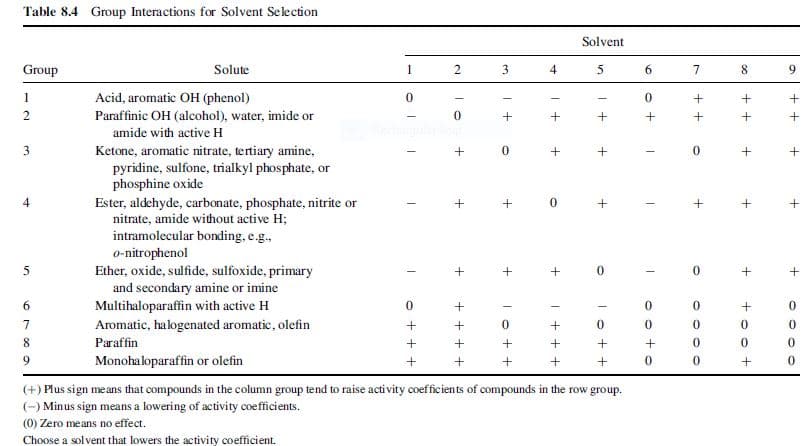
Transcribed Image Text:Table 8.4 Group Interactions for Solvent Selection
Solvent
Group
Solute
1
4
8.
Acid, aromatic OH (phenol)
Paraffinic OH (alcohol), water, imide or
0.
amide with active H
Ketone, aromatic nitrate, tertiary amine,
pyridine, sulfone, trialkyl phosphate, or
phosphine oxide
4
Ester, aldehyde, carbonate, phosphate, nitrite or
nitrate, amide without active H;
intramolecular bonding, e.g.,
o-nitrophenol
Ether, oxide, sulfide, sulfoxide, primary
and secondary amine or imine
Multihaloparaffin with active H
Aromatic, halogenated aromatic, olefin
8.
Paraffin
Monohaloparaffin or olefin
0.
(+) Plus sign means that compounds in the column group tend to raise acti vity coefficients of compounds in the row group.
(-) Minus sign means a lowering of activity coefficients.
(0) Zero means no effect.
Choose a solvent that lowers the activity coefficient.
6.
| + + +
+ + + +
• + + +
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The