Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. A solution containing 3.40 g of sodium carbonate is mixed with one containing 4.86 g of silver nitrate. Part A How many grams of sodium carbonate are present after the reaction is complete? m = Submit Part B Part C X Incorrect; Try Again; 5 attempts remaining m = Submit ΑΣΦ Part D Previous Answers Request Answer How many grams of silver carbonate are present after the reaction is complete? m = | ΑΣΦ ? Request Answer | ΑΣΦ ? g How many grams of sodium nitrate are present after the reaction is complete? ? g 09 g
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. A solution containing 3.40 g of sodium carbonate is mixed with one containing 4.86 g of silver nitrate. Part A How many grams of sodium carbonate are present after the reaction is complete? m = Submit Part B Part C X Incorrect; Try Again; 5 attempts remaining m = Submit ΑΣΦ Part D Previous Answers Request Answer How many grams of silver carbonate are present after the reaction is complete? m = | ΑΣΦ ? Request Answer | ΑΣΦ ? g How many grams of sodium nitrate are present after the reaction is complete? ? g 09 g
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 38CR
Related questions
Question
I need help with part A,C and D. please
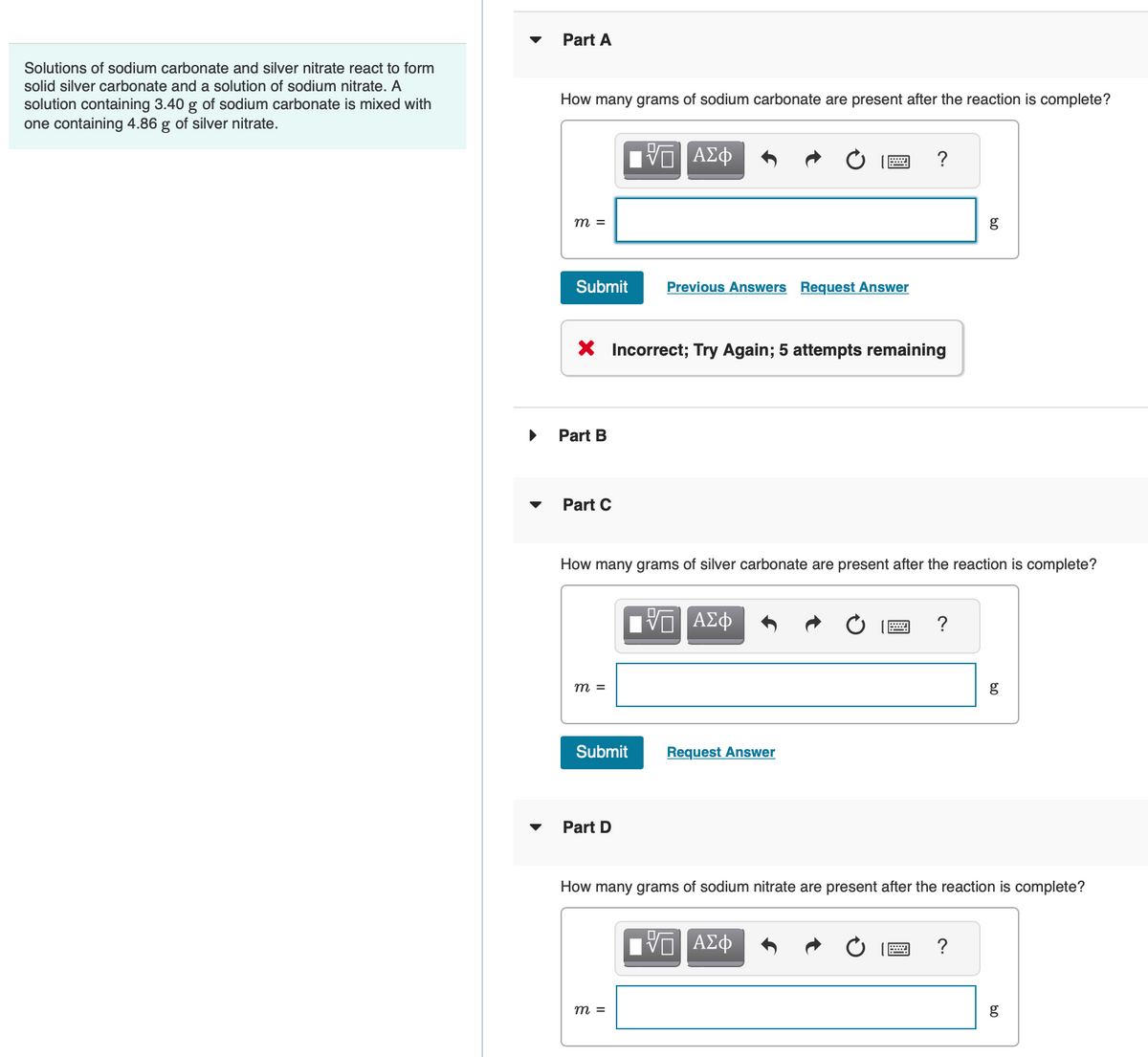
Transcribed Image Text:Solutions of sodium carbonate and silver nitrate react to form
solid silver carbonate and a solution of sodium nitrate. A
solution containing 3.40 g of sodium carbonate is mixed with
one containing 4.86 g of silver nitrate.
Part A
How many grams of sodium carbonate are present after the reaction is complete?
m =
Submit
Part B
Part C
X Incorrect; Try Again; 5 attempts remaining
m =
Submit
ΑΣΦ
Part D
Previous Answers Request Answer
How many grams of silver carbonate are present after the reaction is complete?
m =
| ΑΣΦ
?
Request Answer
| ΑΣΦ
?
g
How many grams of sodium nitrate are present after the reaction is complete?
?
g
09
g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 1 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT