Some chemical compounds are listed in the first column of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. The important chemical species that would be present in this solution are written in the second column of the table. Use the checkboxes to classify each compound. compound NaOH HBr Na Br HE important species present when dissolved in water Na, OH, H₂O H₂O, B.H₂0 Na. Br. H₂O H,O,F.HF, H,O type of compound (check all that apply) weak strong weak acid base base B O Q D □ ionic molecular strong acid O R O THE CO H
Some chemical compounds are listed in the first column of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. The important chemical species that would be present in this solution are written in the second column of the table. Use the checkboxes to classify each compound. compound NaOH HBr Na Br HE important species present when dissolved in water Na, OH, H₂O H₂O, B.H₂0 Na. Br. H₂O H,O,F.HF, H,O type of compound (check all that apply) weak strong weak acid base base B O Q D □ ionic molecular strong acid O R O THE CO H
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section: Chapter Questions
Problem 18PS: Some lithium chloride, LiCl, is dissolved in 100 mL of water in one beaker, and some Li2SO4 is...
Related questions
Question
Please do it neat and clean correctly on a plain white paper
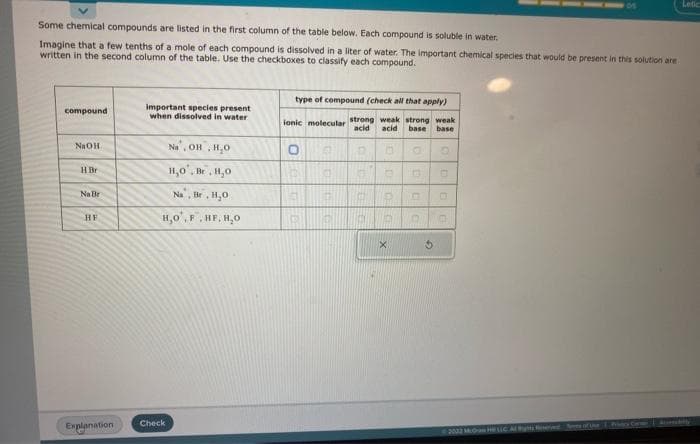
Transcribed Image Text:Some chemical compounds are listed in the first column of the table below. Each compound is soluble in water.
Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. The important chemical species that would be present in this solution are
written in the second column of the table. Use the checkboxes to classify each compound.
compound
NaOH
HBr
Na Br
HE
Explanation
important species present
when dissolved in water
Check
Na, OH, H₂O
H₂O, Br,H,O
Na, Br, H₂0
H,O, F, HF, H₂O
type of compound (check all that apply)
ionic molecular strong weak strong weak
acid acid base base
O
PO
O
0
A
a
DOO
X
DCO
G
E
2032 Maw LLC Ass of Use
Letic
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
