Some chemical compounds are listed in the first column of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. The important chemical species that would be present in this solution are written in the second column of the table. Use the checkboxes to classify each compound. type of compound (check all that apply) compound Important species present when dissolved in water lonic molecular strong weak strong weak acid acid base base 2+ Ba(OH)₂ Ba, OH, H₂O 0 CHẠNH. C₂H₂NH, OH, C₂H₂NH₂, H₂O ΚΙ K², 1, H₂O NaOH Na, OH, H₂O DO 000 0 0 0 0 0 0 0 0 0 X Ś 0 ?
Some chemical compounds are listed in the first column of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. The important chemical species that would be present in this solution are written in the second column of the table. Use the checkboxes to classify each compound. type of compound (check all that apply) compound Important species present when dissolved in water lonic molecular strong weak strong weak acid acid base base 2+ Ba(OH)₂ Ba, OH, H₂O 0 CHẠNH. C₂H₂NH, OH, C₂H₂NH₂, H₂O ΚΙ K², 1, H₂O NaOH Na, OH, H₂O DO 000 0 0 0 0 0 0 0 0 0 X Ś 0 ?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section: Chapter Questions
Problem 18PS: Some lithium chloride, LiCl, is dissolved in 100 mL of water in one beaker, and some Li2SO4 is...
Related questions
Question
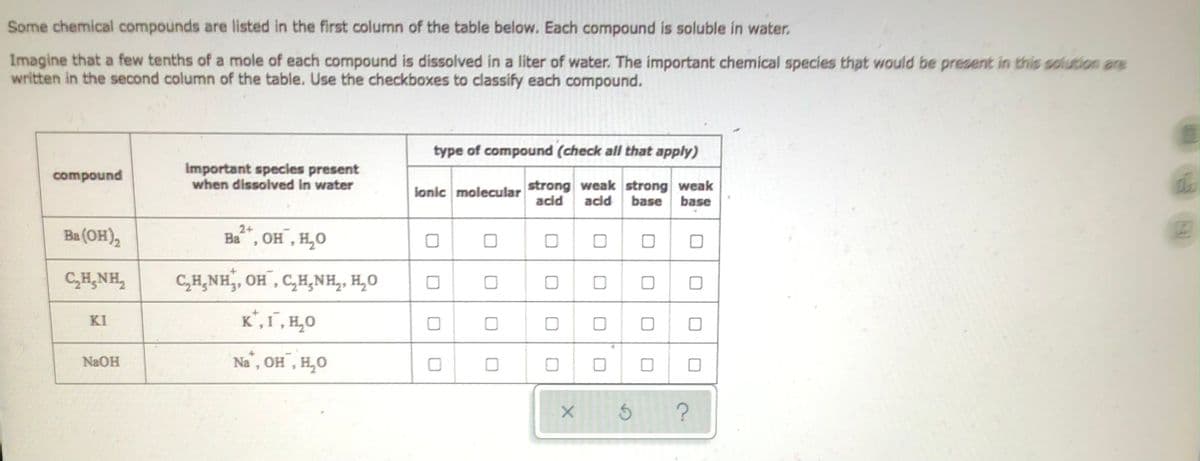
Transcribed Image Text:Some chemical compounds are listed in the first column of the table below. Each compound is soluble in water.
Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. The important chemical species that would be present in this solution are
written in the second column of the table. Use the checkboxes to classify each compound.
type of compound (check all that apply)
compound
Important species present
when dissolved in water
ionic molecular
strong weak strong weak
acid acid base base
2+
Ba(OH)₂
Ba, OH, H₂O
0
0
CHẠNH,
C₂H₂NH₂, OH, C₂H₂NH₂, H₂O
но
0
0
0
0
+
ΚΙ
K, I, H₂O
NaOH
Na, OH, H₂O
0
0
X
□
3
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT