Some chlorine atoms have an atomic mass of 37, while others have an atomic mass of 35. What is the difference between the two types of chlorine atom?
Some chlorine atoms have an atomic mass of 37, while others have an atomic mass of 35. What is the difference between the two types of chlorine atom?
Chapter3: Atoms And Elements
Section: Chapter Questions
Problem 59E: When we refer to doughnuts or cookies, we often refer to 1doz of them, which corresponds to 12. Why...
Related questions
Question
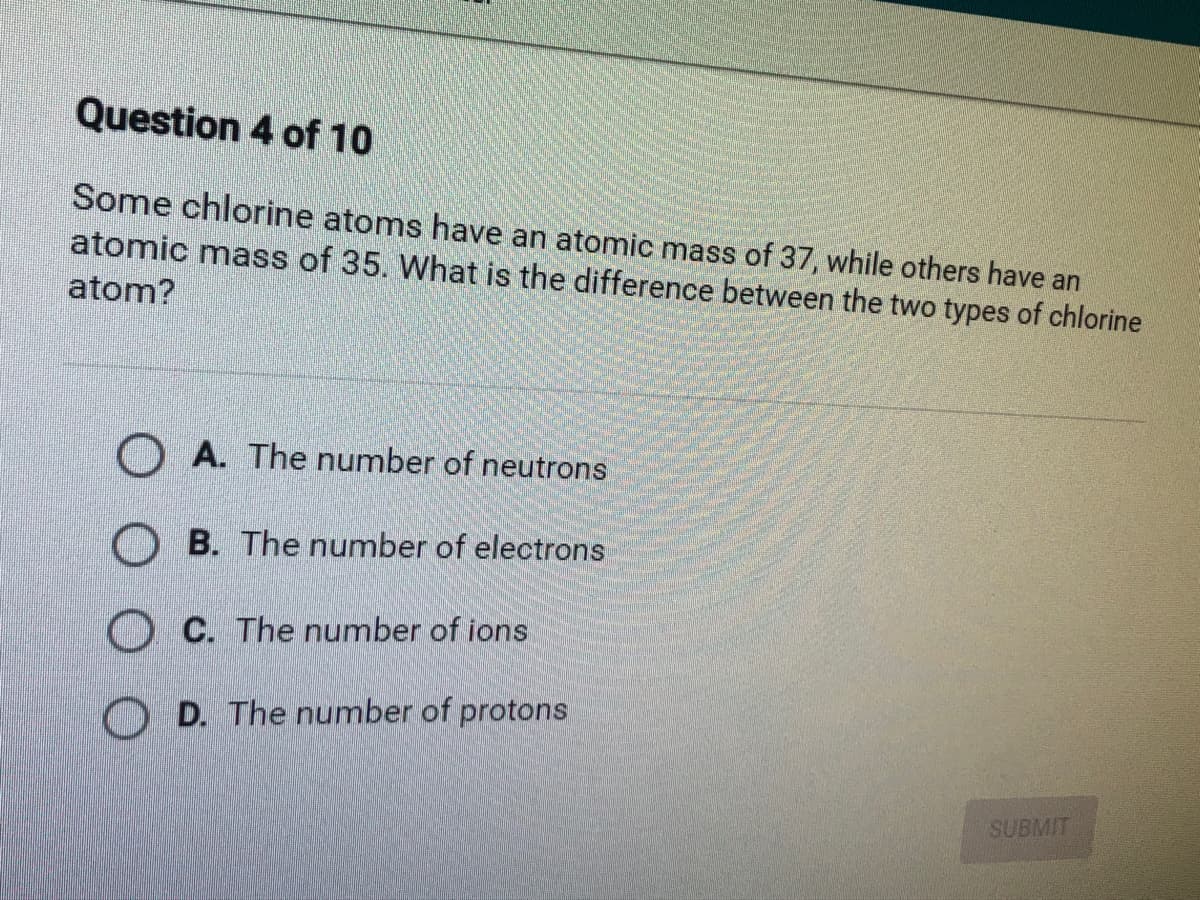
Transcribed Image Text:Question 4 of 10
Some chlorine atoms have an atomic mass of 37, while others have an
atomic mass of 35. What is the difference between the two types of chlorine
atom?
O A. The number of neutrons
O B. The number of electrons
O C. The number of ions
D. The number of protons
SUBMIT
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning