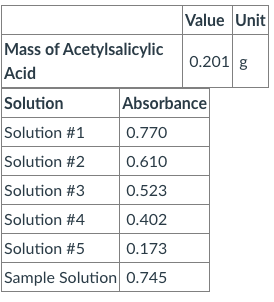
Standard solution : 0.201 grams of acetylsalicylic acid was combined with 10 mL of 0.5 M NaOH, then transfered to a 100 mL flask and filled the rest of the way with water Then 0.500 mLs of the standard solution was transfered to a 10.00 mL flask and diluted to the 10 mL mark with 0.02 M of buffered iron chloride. This step was repeated using 0.400, 0.300, 0.200, 0.100 mLs of the standard solution Questions: A.) determine the concentration of the acetylsalicylic acid in the 100.00 mL volumetric flask containing the solution from which the standards were made. B.)determine the concentration of the acetylsalicylic acid in each of the 10.00 mL calibration solutions. I only need the work for 0.400 mLs C.)Make a scatter plot (on the computer again) of absorbance versus concentration for the calibration samples. On the plot, add a linear trendline, and display the equation and the R2 value on the chart. Take your time to label the axes
Standard solution : 0.201 grams of acetylsalicylic acid was combined with 10 mL of 0.5 M NaOH, then transfered to a 100 mL flask and filled the rest of the way with water
Then 0.500 mLs of the standard solution was transfered to a 10.00 mL flask and diluted to the 10 mL mark with 0.02 M of buffered iron chloride. This step was repeated using 0.400, 0.300, 0.200, 0.100 mLs of the standard solution
Questions:
A.) determine the concentration of the acetylsalicylic acid in the 100.00 mL volumetric flask containing the solution from which the standards were made.
B.)determine the concentration of the acetylsalicylic acid in each of the 10.00 mL calibration solutions. I only need the work for 0.400 mLs
C.)Make a scatter plot (on the computer again) of absorbance versus concentration for the calibration samples. On the plot, add a linear trendline, and display the equation and the R2 value on the chart. Take your time to label the axes
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images









