State whether it is possible to determine whether one polymer is more likely to crystallize than the other. If it is possible, note which is the more likely and then cite reason(s) for your choice. If it is not possible to decide, state why. a) Linear and syndiotactic poly(vinyl chloride); linear and isotactic polystyrene b) Network phenol-formaldehyde; linear and heavily crosslinked cis-isoprene c) Linear polyethylene; lightly branched isotactic polypropylene d) Alternating poly(styrene-ethylene) copolymer; random poly(vinyl chloridetetrafluoroethylene) copolymer
State whether it is possible to determine whether one polymer is more likely to crystallize than the other. If it is possible, note which is the more likely and then cite reason(s) for your choice. If it is not possible to decide, state why. a) Linear and syndiotactic poly(vinyl chloride); linear and isotactic polystyrene b) Network phenol-formaldehyde; linear and heavily crosslinked cis-isoprene c) Linear polyethylene; lightly branched isotactic polypropylene d) Alternating poly(styrene-ethylene) copolymer; random poly(vinyl chloridetetrafluoroethylene) copolymer
Chapter31: Synthetic Polymers
Section31.SE: Something Extra
Problem 19MP
Related questions
Question
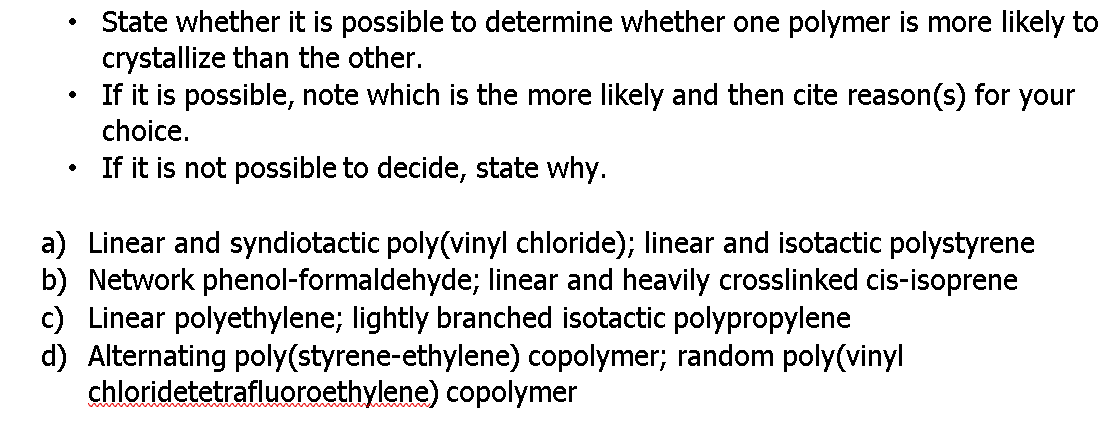
Transcribed Image Text:State whether it is possible to determine whether one polymer is more likely to
crystallize than the other.
If it is possible, note which is the more likely and then cite reason(s) for your
choice.
If it is not possible to decide, state why.
a) Linear and syndiotactic poly(vinyl chloride); linear and isotactic polystyrene
b) Network phenol-formaldehyde; linear and heavily crosslinked cis-isoprene
c) Linear polyethylene; lightly branched isotactic polypropylene
d) Alternating poly(styrene-ethylene) copolymer; random poly(vinyl
chloridetetrafluoroethylene) copolymer
wwaw m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT