step 1 elementary reaction NO₂(9) NO(g) + O(g) 1 k₁ 2 O(g) + NO₂(g) → O₂(g) + NO(g) 1 k₂ Suppose also k₁k2. That is, the first step is much faster than the second. Write the balanced chemical equation for the overall chemical reaction: Write the experimentaily- observable rate law for the overall chemical reaction. Note: your answer should not contain the concentrations of any intermediates. Express the rate constant k for the overall chemical reaction in terms of k₁, K₂, and (if necessary) the rate constants k.1 and k.2 for the reverse of the two elementary reactions in the mechanism. 0 rate=& rate constant k = 0 ローロ Olo 8 X
step 1 elementary reaction NO₂(9) NO(g) + O(g) 1 k₁ 2 O(g) + NO₂(g) → O₂(g) + NO(g) 1 k₂ Suppose also k₁k2. That is, the first step is much faster than the second. Write the balanced chemical equation for the overall chemical reaction: Write the experimentaily- observable rate law for the overall chemical reaction. Note: your answer should not contain the concentrations of any intermediates. Express the rate constant k for the overall chemical reaction in terms of k₁, K₂, and (if necessary) the rate constants k.1 and k.2 for the reverse of the two elementary reactions in the mechanism. 0 rate=& rate constant k = 0 ローロ Olo 8 X
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 112QRT
Related questions
Question
❤️❤️??helpppppp
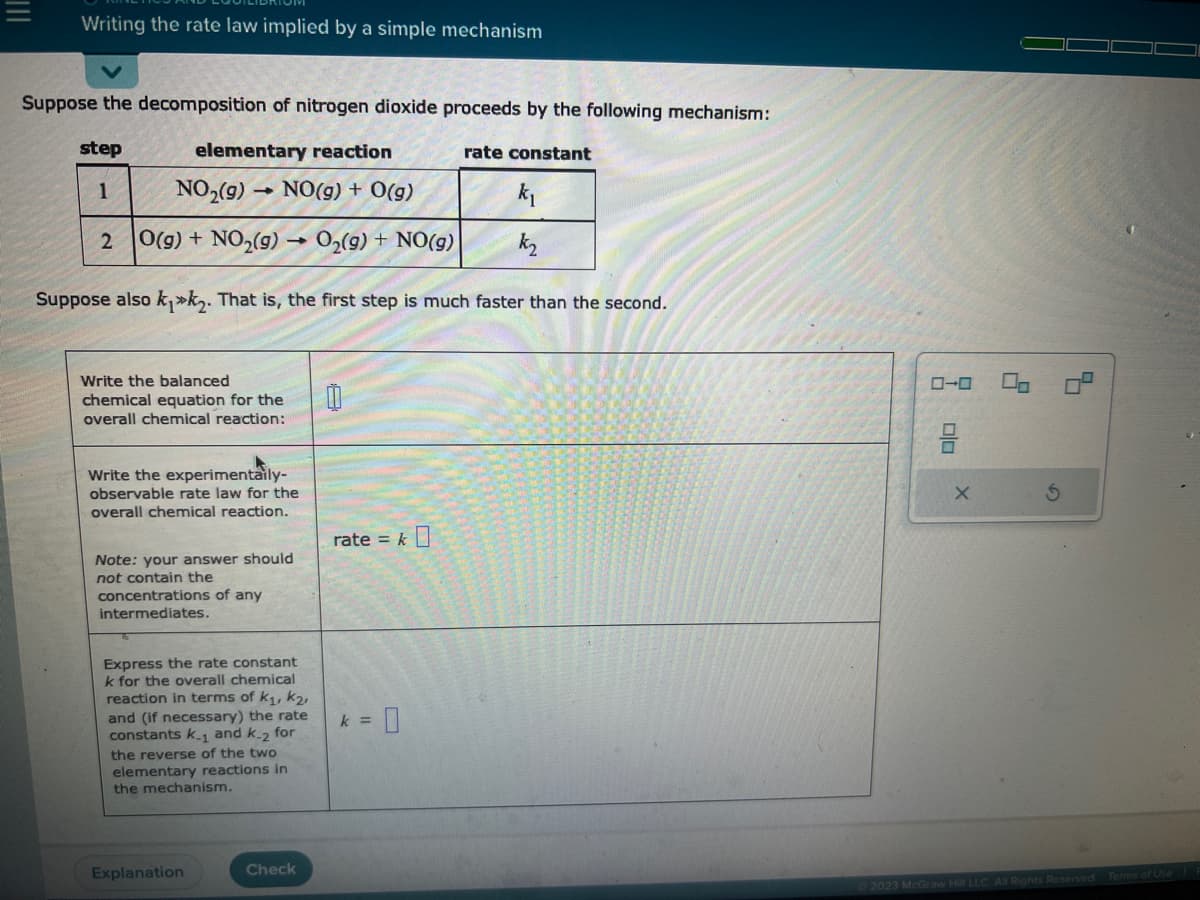
Transcribed Image Text:Writing the rate law implied by a simple mechanism
Suppose the decomposition of nitrogen dioxide proceeds by the following mechanism:
elementary reaction
step
1
NO₂(g)
NO(g) + 0(g)
k₁
O(g) + NO₂(g) →O₂(g) + NO(g)
k₂
Suppose also k₁k₂. That is, the first step is much faster than the second.
2
1
Write the balanced
chemical equation for the
overall chemical reaction:
Write the experimentaily-
observable rate law for the
overall chemical reaction.
Note: your answer should
not contain the
concentrations of any
intermediates.
Express the rate constant
k for the overall chemical
reaction in terms of k₁, K₂,
and (if necessary) the rate
constants k.1 and k-2 for
the reverse of the two
elementary reactions in
the mechanism.
Explanation
Check
0
rate = k
= 0
rate constant
k =
00
2023 McGraw Hill LLC. All Rights Reserved. Terms of Use P
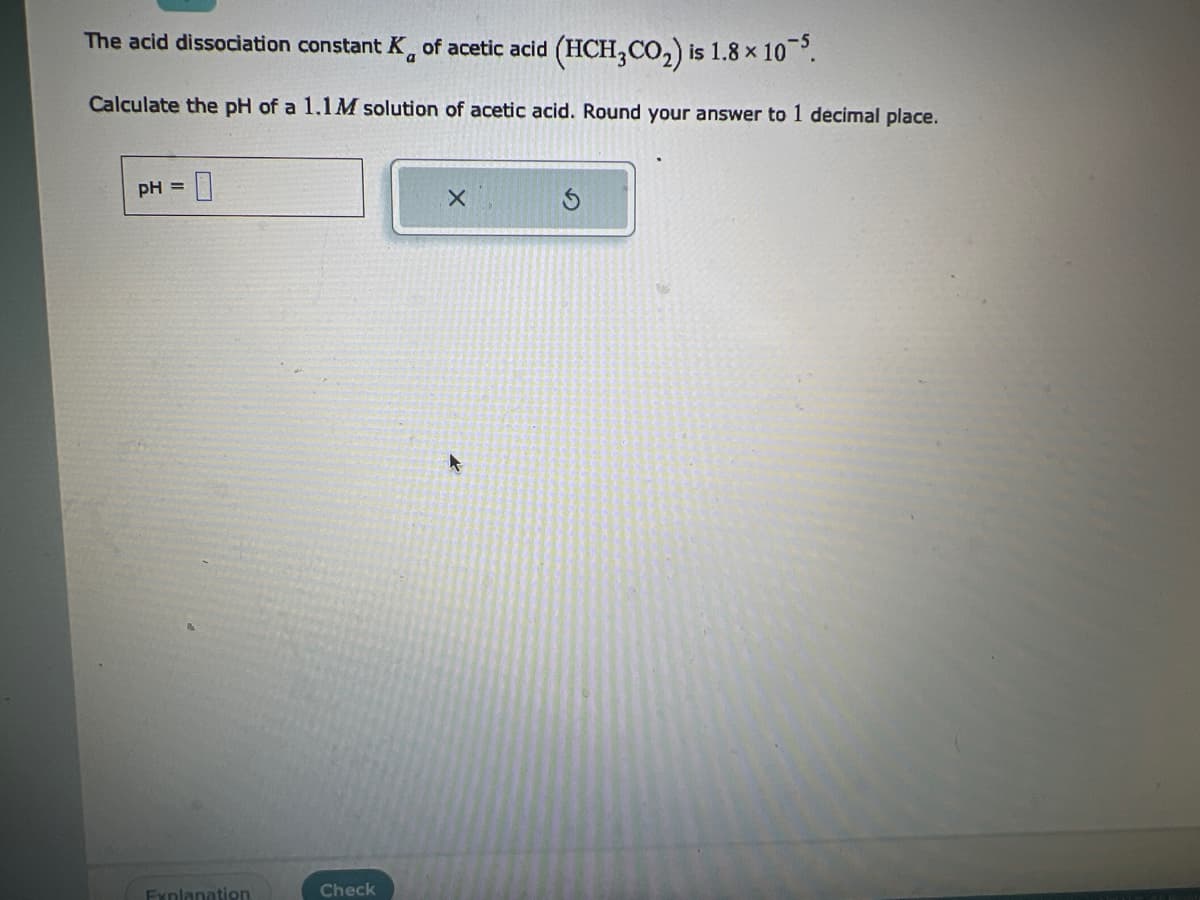
Transcribed Image Text:The acid dissociation constant K of acetic acid (HCH,CO₂) is 1.8 x 10-5.
Calculate the pH of a 1.1M solution of acetic acid. Round your answer to 1 decimal place.
pH = 0
Explanation
Check
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning