steps). a. hydration with mercury as catalyst b. oxidation with potassium permanganate c. hydrohalogenation with HCI (only one HCl is given) d. hydration with borane e. halogenation with bromine (2 molecules of bromine are given) f. hydrogenation with Lindlar catalyst g. hydrogenation with Li/NH3 h. hydrogenation with Pt/C
steps). a. hydration with mercury as catalyst b. oxidation with potassium permanganate c. hydrohalogenation with HCI (only one HCl is given) d. hydration with borane e. halogenation with bromine (2 molecules of bromine are given) f. hydrogenation with Lindlar catalyst g. hydrogenation with Li/NH3 h. hydrogenation with Pt/C
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.8: Oxidation Of Aromatic Compounds
Problem 20P
Related questions
Question
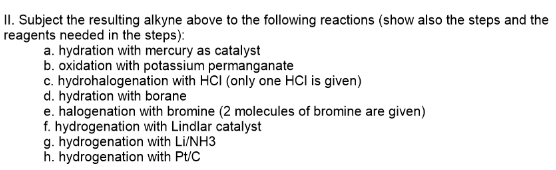
Transcribed Image Text:II. Subject the resulting alkyne above to the following reactions (show also the steps and the
reagents needed in the steps):
a. hydration with mercury as catalyst
b. oxidation with potassium permanganate
c. hydrohalogenation with HCI (only one HCl is given)
d. hydration with borane
e. halogenation with bromine (2 molecules of bromine are given)
f. hydrogenation with Lindlar catalyst
g. hydrogenation with Li/NH3
h. hydrogenation with Pt/C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

