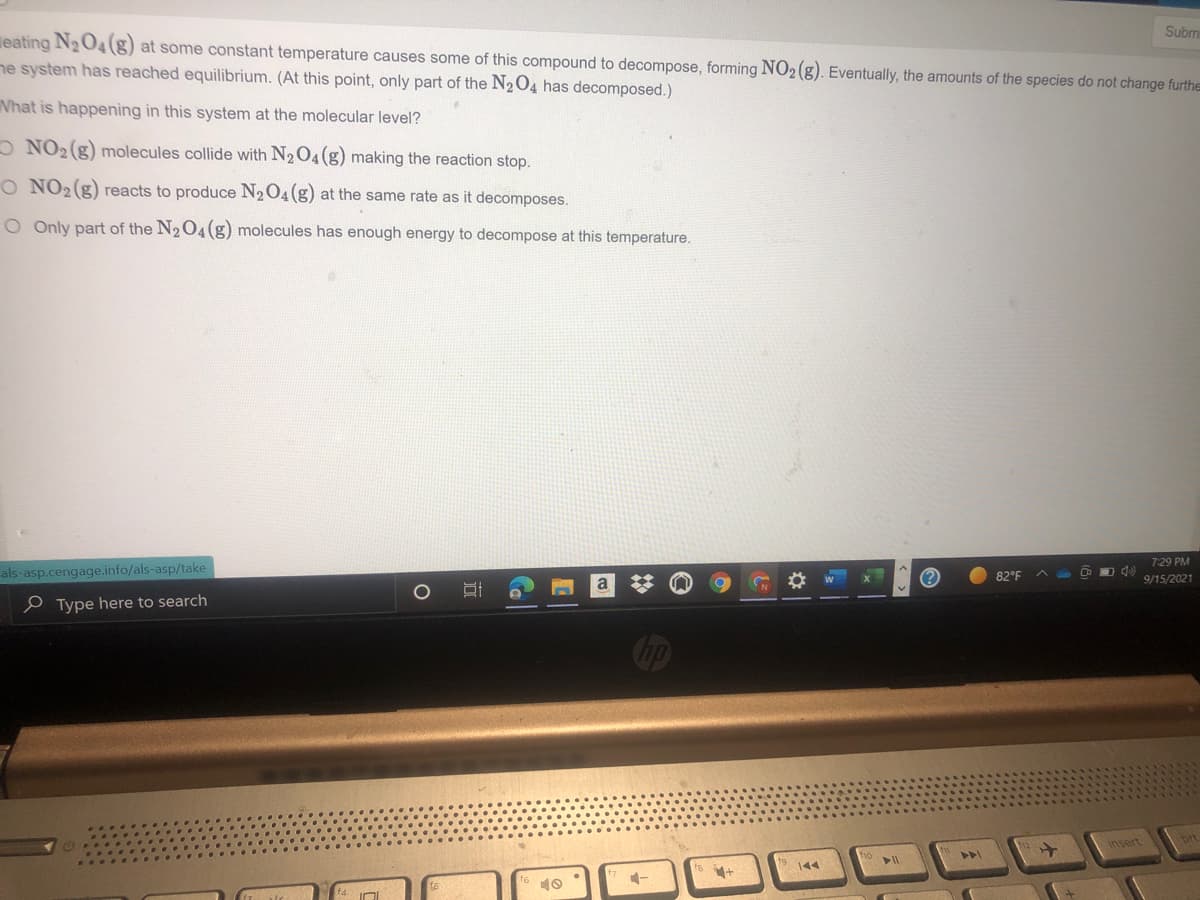
Sub leating N2O4(g) at some constant temperature causes some of this compound to decompose, forming NO2(g). Eventually, the amounts of the species do not change furt ne system has reached equilibrium. (At this point, only part of the N204 has decomposed.) What is happening in this system at the molecular level? O NO2 (g) molecules collide with N2O4 (g) making the reaction stop. O NO2(g) reacts to produce N204(g) at the same rate as it decomposes. O Only part of the N204(g) molecules has enough energy to decompose at this temperature.
Sub leating N2O4(g) at some constant temperature causes some of this compound to decompose, forming NO2(g). Eventually, the amounts of the species do not change furt ne system has reached equilibrium. (At this point, only part of the N204 has decomposed.) What is happening in this system at the molecular level? O NO2 (g) molecules collide with N2O4 (g) making the reaction stop. O NO2(g) reacts to produce N204(g) at the same rate as it decomposes. O Only part of the N204(g) molecules has enough energy to decompose at this temperature.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter18: Thermodynamics And Equilibrium
Section: Chapter Questions
Problem 18.111QP: Adenosine triphosphate, ATP, is used as a free-energy source by biological cells. (See the essay on...
Related questions
Question
Transcribed Image Text:Subm
leating N2O4(g) at some constant temperature causes some of this compound to decompose, forming NO2(g). Eventually, the amounts of the species do not change furthe
ne system has reached equilibrium. (At this point, only part of the N204 has decomposed.)
What is happening in this system at the molecular level?
O NO2 (g) molecules collide with N2O4(g) making the reaction stop.
O NO2(g) reacts to produce N204(g) at the same rate as it decomposes.
O Only part of the N2O4(g) molecules has enough energy to decompose at this temperature.
7:29 PM
als-asp.cengage.info/als-asp/take
82°F
9/15/2021
e Type here to search
insert
144
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning