Submit a clean, dry, and properly labeled 50-mL reagent bottle for your unknown solution. Pipet 20.00 mL of the sample in 250-mL Erlenmeyer flask. Add 5 mL of buffer and 5 drops of indicator. Titrate the solution until it turns light blue. If the titration consumes more than 50 mL of the titrant, dilute the sample accordingly. Compute for ppm of CaCO3 using the following table. Mean Molarity = 2.487 x 10^-3 M Blank correction = 0.015
Submit a clean, dry, and properly labeled 50-mL reagent bottle for your unknown solution. Pipet 20.00 mL of the sample in 250-mL Erlenmeyer flask. Add 5 mL of buffer and 5 drops of indicator. Titrate the solution until it turns light blue. If the titration consumes more than 50 mL of the titrant, dilute the sample accordingly. Compute for ppm of CaCO3 using the following table. Mean Molarity = 2.487 x 10^-3 M Blank correction = 0.015
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter13: Gases
Section: Chapter Questions
Problem 117A
Related questions
Question
Submit a clean, dry, and properly labeled 50-mL reagent bottle for your unknown solution. Pipet 20.00 mL of the sample in 250-mL Erlenmeyer flask. Add 5 mL of buffer and 5 drops of indicator. Titrate the solution until it turns light blue. If the titration consumes more than 50 mL of the titrant, dilute the sample accordingly. Compute for ppm of CaCO3 using the following table.
Mean Molarity = 2.487 x 10^-3 M
Blank correction = 0.015
Formula = V (mL) titrant x Mean Molarity of Titrant x Molecular weight of CaCO3 x 1/V (L) sample
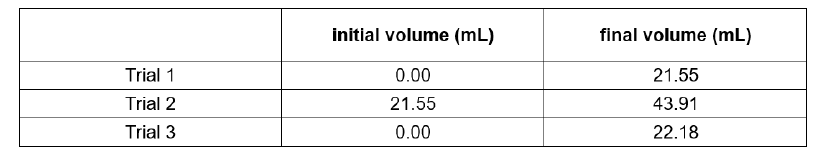
Transcribed Image Text:initial volume (mL)
final volume (mL)
Trial 1
0.00
21.55
Trial 2
21.55
43.91
Trial 3
0.00
22.18
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co
