Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter7: Chemical Bonding And Molecular Geometry
Section: Chapter Questions
Problem 112E: Use the simulation (http://openstaxcollege.org/l/16MolecPolarity) to perform the following exercises...
Related questions
Question
Subparts number 4 and 5
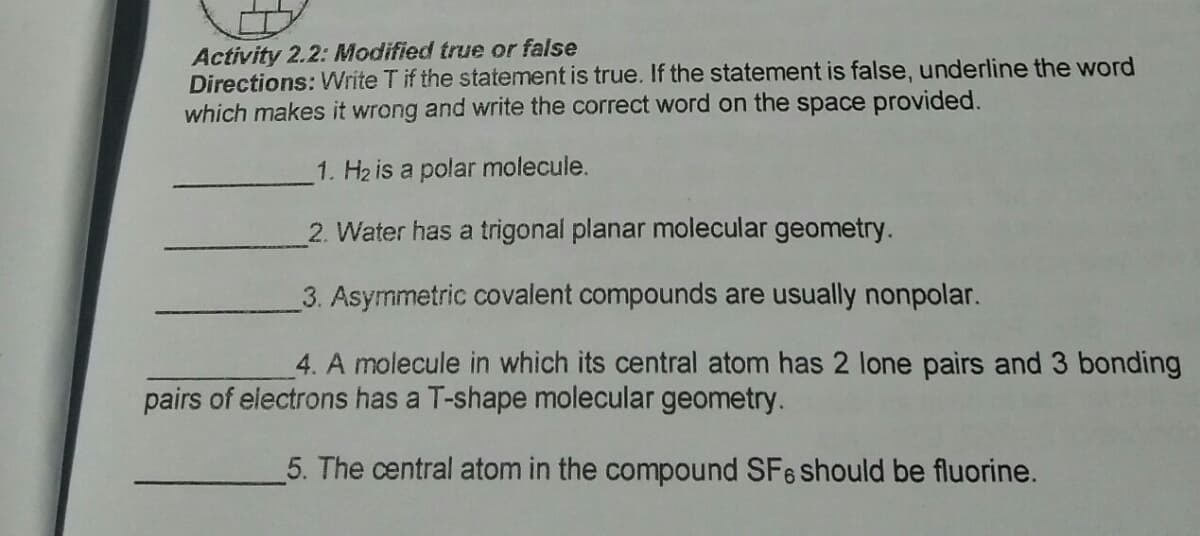
Transcribed Image Text:Activity 2.2: Modified true or false
Directions: Write T if the statement is true. If the statement is false, underline the word
which makes it wrong and write the correct word on the space provided.
1. H2 is a polar molecule.
2. Water has a trigonal planar molecular geometry.
3. Asymmetric covalent compounds are usually nonpolar.
4. A molecule in which its central atom has 2 lone pairs and 3 bonding
pairs of electrons has a T-shape molecular geometry.
5. The central atom in the compound SFe should be fluorine.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole