Sulfuric acid is an important industrial chemical that is usually produced by a series of reactions. One of these involves an equilibrium between gaseous sulfur dioxide, oxygen, and sulfur trioxide. 2 SO,(g) + 0,(g) 2 SO,(g) If 2.5 mol of sulfur dioxide gas and 2.0 mol of oxygen gas are placed in a sealed 1.0 L container and allowed to reach equilibrium, 0.75 mol of sulfur dioxide remains. Use an ICE table to determine the concentration of the other gases at equilibrium.
Sulfuric acid is an important industrial chemical that is usually produced by a series of reactions. One of these involves an equilibrium between gaseous sulfur dioxide, oxygen, and sulfur trioxide. 2 SO,(g) + 0,(g) 2 SO,(g) If 2.5 mol of sulfur dioxide gas and 2.0 mol of oxygen gas are placed in a sealed 1.0 L container and allowed to reach equilibrium, 0.75 mol of sulfur dioxide remains. Use an ICE table to determine the concentration of the other gases at equilibrium.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 90QRT
Related questions
Question
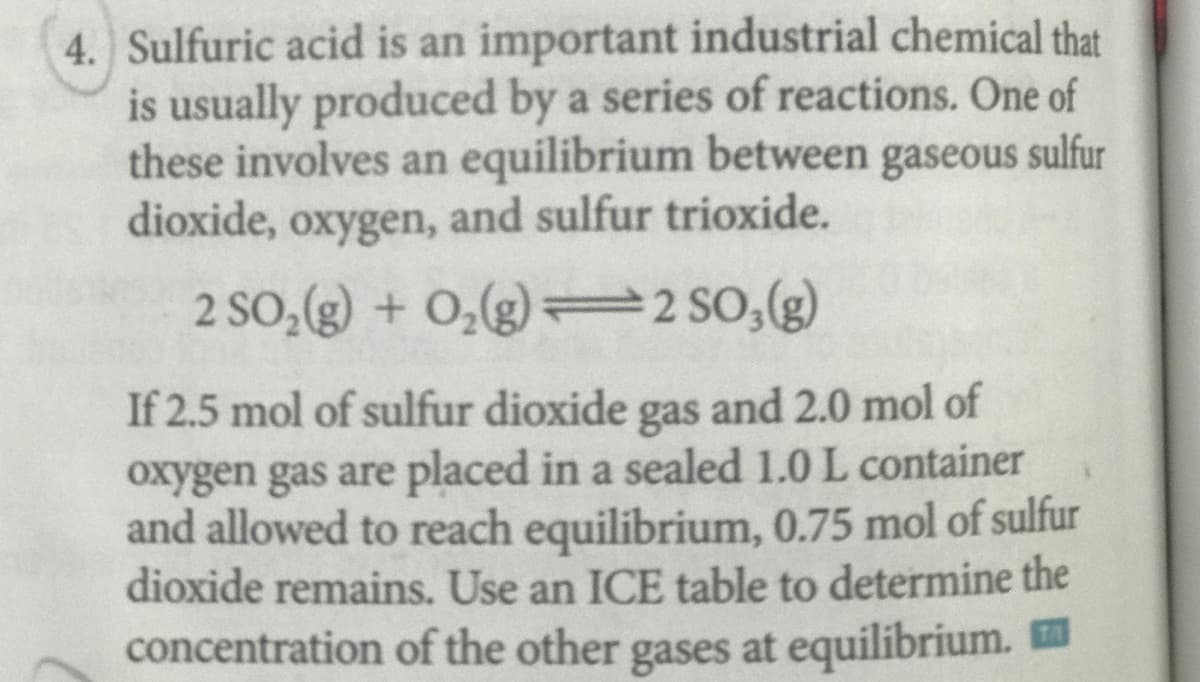
Transcribed Image Text:4. Sulfuric acid is an important industrial chemical that
is usually produced by a series of reactions. One of
these involves an equilibrium between gaseous sulfur
dioxide, oxygen, and sulfur trioxide.
2 SO,(g) + O,(g) = 2 SO,(g)
If 2.5 mol of sulfur dioxide gas and 2.0 mol of
oxygen gas are placed in a sealed 1.0 L container
and allowed to reach equilibrium, 0.75 mol of sulfur
dioxide remains. Use an ICE table to determine the
concentration of the other gases at equilibrium. E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning