SUPPLEMENT: The human body is 0.4% potassium by weight. 0.0117% of all potassium is 4°K. Calculate the rate of decay (dpm) experienced by the average person due to 4°K within their own body. Assume an average weight of 155 lb (70.5 kg). person's weight in grams grams K g grams 40K g moles 4°K moles atoms 4°K atoms 40K beta decay rate constant min- k = ( In 2 ) / ( t,2) decay rate of 40K in body dpm dpm = (k )( atoms 4°K ) This is a much higher decay rate that was produced by the sample of compound in the experiment. But if the probe were held next to your body, practically none would be detected. Explain why.
SUPPLEMENT: The human body is 0.4% potassium by weight. 0.0117% of all potassium is 4°K. Calculate the rate of decay (dpm) experienced by the average person due to 4°K within their own body. Assume an average weight of 155 lb (70.5 kg). person's weight in grams grams K g grams 40K g moles 4°K moles atoms 4°K atoms 40K beta decay rate constant min- k = ( In 2 ) / ( t,2) decay rate of 40K in body dpm dpm = (k )( atoms 4°K ) This is a much higher decay rate that was produced by the sample of compound in the experiment. But if the probe were held next to your body, practically none would be detected. Explain why.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter18: Nuclear Chemistry
Section: Chapter Questions
Problem 18.ACP
Related questions
Question
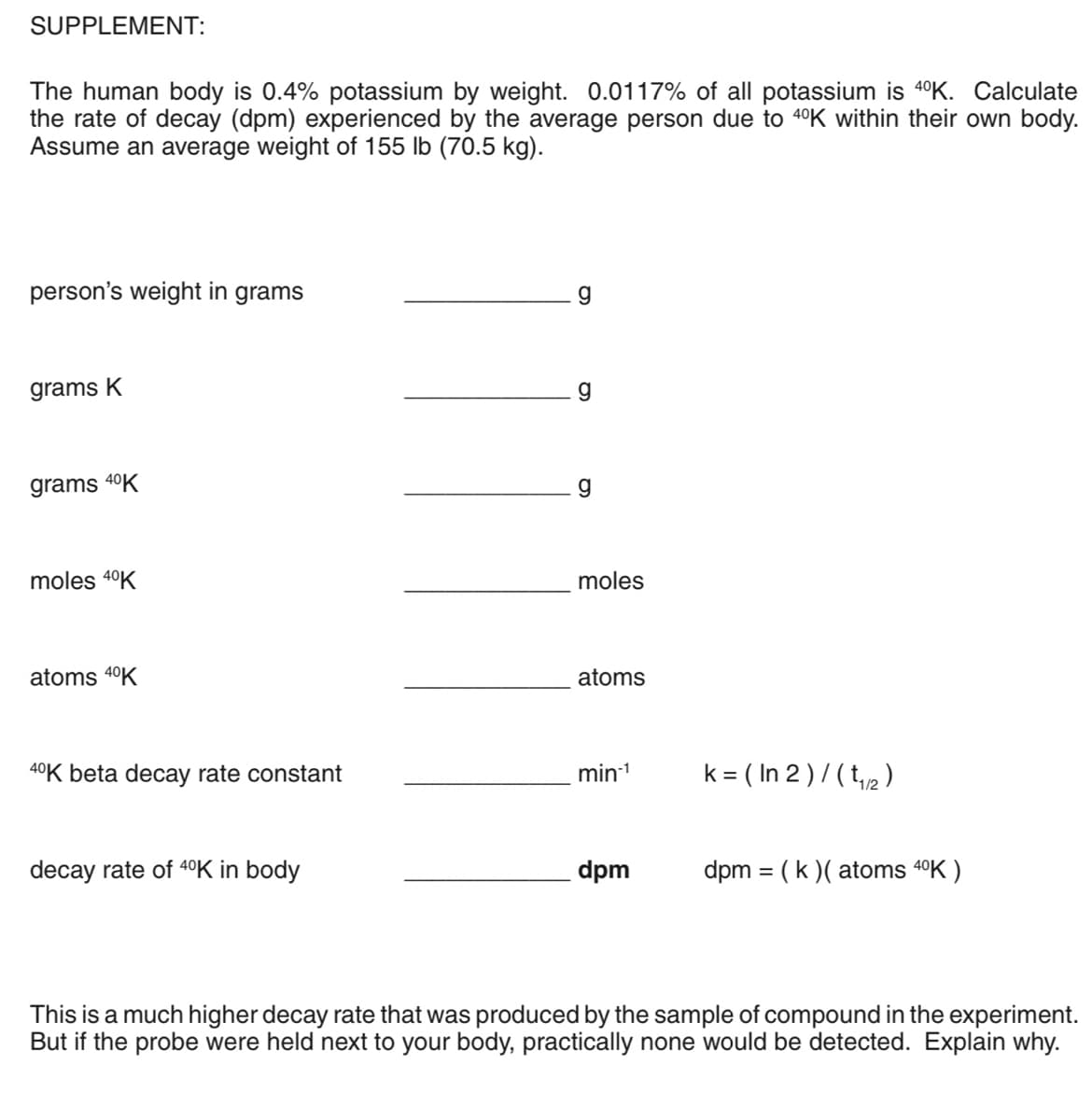
Transcribed Image Text:SUPPLEMENT:
The human body is 0.4% potassium by weight. 0.0117% of all potassium is 4°K. Calculate
the rate of decay (dpm) experienced by the average person due to 40K within their own body.
Assume an average weight of 155 lb (70.5 kg).
person's weight in grams
g
grams K
grams 40K
g
moles 4°K
moles
atoms 40K
atoms
40K beta decay rate constant
min-1
k = ( In 2 ) / ( t,2 )
decay rate of 40K in body
dpm
dpm = (k )( atoms 40K )
%3D
This is a much higher decay rate that was produced by the sample of compound in the experiment.
But if the probe were held next to your body, practically none would be detected. Explain why.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
