Suppose 6.93 g of solid Mg(OH)2 is added to 27.1 mL of 0.510 M H2SO4 solution. What will the concentration of Mg2+ be when all of the acid has been neutralized? i M How many grams of Mg(OH)2 will not have dissolved? eTextbook and Media Attempts: 0 of 15 used Submit Answer Save for Later
Suppose 6.93 g of solid Mg(OH)2 is added to 27.1 mL of 0.510 M H2SO4 solution. What will the concentration of Mg2+ be when all of the acid has been neutralized? i M How many grams of Mg(OH)2 will not have dissolved? eTextbook and Media Attempts: 0 of 15 used Submit Answer Save for Later
Chapter4: Calculations Used In Analytical Chemistry
Section: Chapter Questions
Problem 4.24QAP
Related questions
Question
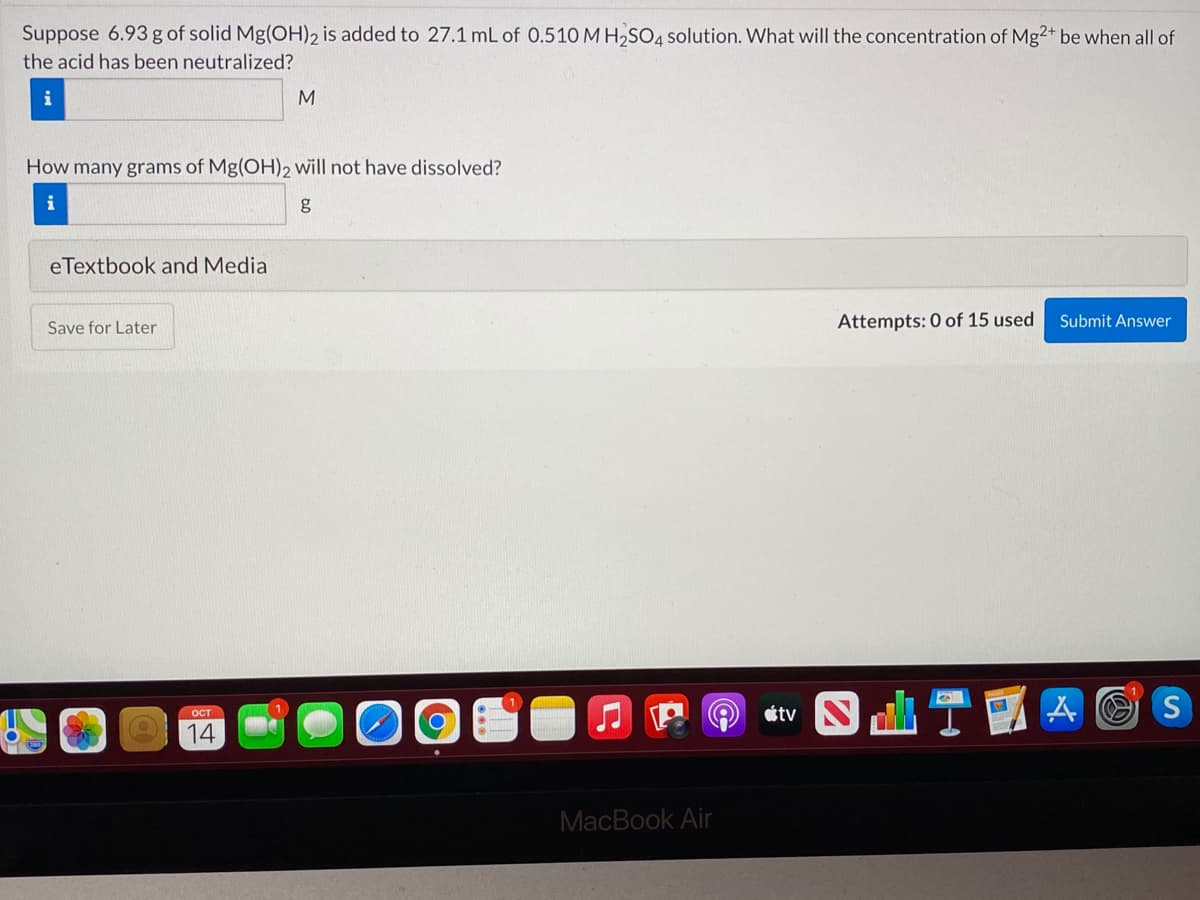
Transcribed Image Text:Suppose 6.93 g of solid Mg(OH)2 is added to 27.1 mL of 0.510 M H2SO4 solution. What will the concentration of Mg2+ be when all of
the acid has been neutralized?
i
M
How many grams of Mg(OH)2 will not have dissolved?
eTextbook and Media
Attempts: 0 of 15 used
Submit Answer
Save for Later
étv N
ITE
OCT
14
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning