Suppose that an experiment required that 250 ml of a 35 ppm aqueous dipotassium phosphate (K2HPO4) solution be prepared from a 0.1 M stock K2HPO4 solution prepared earlier. What volume of the concentrated KHPO4 solution would be needed? (Water density = 997 g/L at 25°C and MW of KHPO4 = 174 g/mol) %3D
Suppose that an experiment required that 250 ml of a 35 ppm aqueous dipotassium phosphate (K2HPO4) solution be prepared from a 0.1 M stock K2HPO4 solution prepared earlier. What volume of the concentrated KHPO4 solution would be needed? (Water density = 997 g/L at 25°C and MW of KHPO4 = 174 g/mol) %3D
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.107E
Related questions
Question
R1
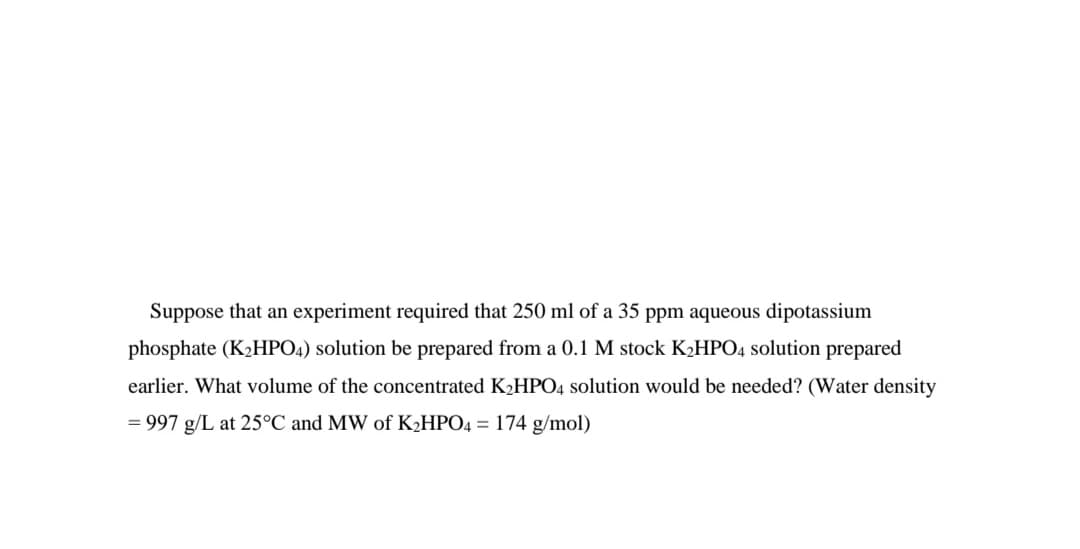
Transcribed Image Text:Suppose that an experiment required that 250 ml of a 35 ppm aqueous dipotassium
phosphate (K2HPO4) solution be prepared from a 0.1 M stock K2HPO4 solution prepared
earlier. What volume of the concentrated K2HPO4 solution would be needed? (Water density
= 997 g/L at 25°C and MW of KHPO4 = 174 g/mol)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning