Switched on, the water temperature reached a value 0 = 60 °C within a inside the container, which can supply heat at constant rate, is boiling? Boiling point of water is 6, = 100 °C. y vessel contains V, = 20 L water at temperature 6: 20 °C. When a %3D heater %3D 240 min AUf instead of water, some other liquid having all properties same as those of water except the boiling point that is 150 °C, is used, how Jong after the beginning of the experiment, will the mixture start boiling? The liquid is miscible in water. nerev
Switched on, the water temperature reached a value 0 = 60 °C within a inside the container, which can supply heat at constant rate, is boiling? Boiling point of water is 6, = 100 °C. y vessel contains V, = 20 L water at temperature 6: 20 °C. When a %3D heater %3D 240 min AUf instead of water, some other liquid having all properties same as those of water except the boiling point that is 150 °C, is used, how Jong after the beginning of the experiment, will the mixture start boiling? The liquid is miscible in water. nerev
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter16: Temperature And The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 50P
Related questions
Question
Kindly check the answer in picture containing the question before submitting the solution the solution.
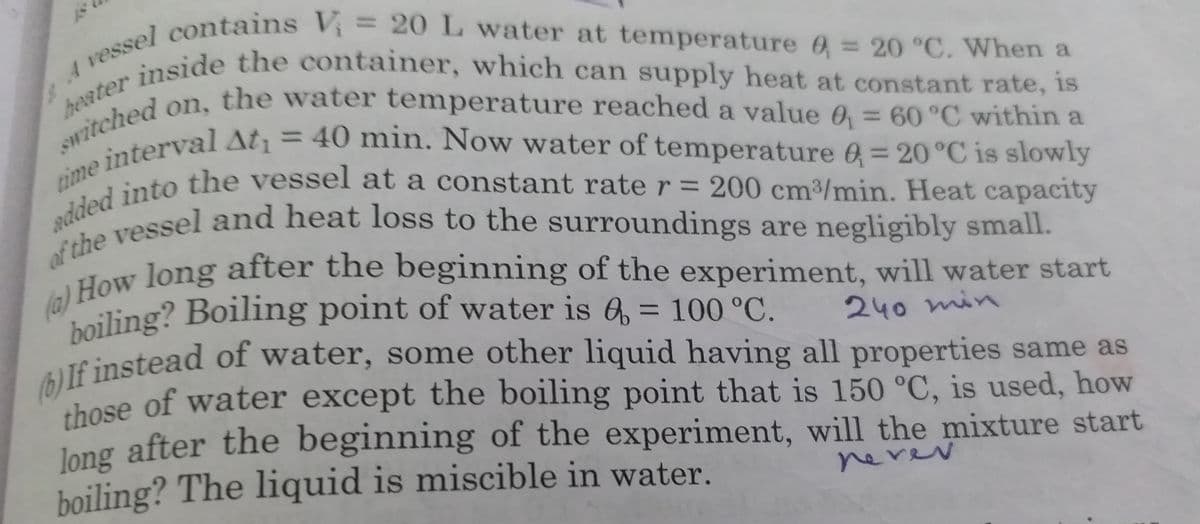
Transcribed Image Text:time interval At¡ = 40 min. Now water of temperature 6 = 20°C is slowly
inside the container, which can supply heat at constant rate, is
vessside the container, which can supply heat at constant rate, is
switched on, the water temperature reached a value 0 = 60 °C within a
boiling? Boiling point of water is 6, = 100 °C.
F 1 contains V= 20L water at temperature 6 = 20 °C. When a
%3D
on, the water temperature reached a value 0 = 60 °C within a
heater
%3D
%3D
200 cm3/min. Heat capacity
adaeessel and heat loss to the surroundings are negligibly small.
%3D
dow long after the beginning of the experiment, will water start
boiling? Boiling point of water is 6, = 100 °C.
If instead of water, some other liquid having all properties same as
shose of water except the boiling point that is 150 °C, is used, how
240 min
thosec
Jong after the beginning of the experiment, will the mixture start
boiling? The liquid is miscible in water.
nevev
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning