T Questions 1-2: KC103 2 KCI+ 02 1. How many grams of O2 are produced from the decomposition of 8.56 grams of KC1032 2. How many grams of potassium chloride, KCl are produced if 13.2g of potassium chlorate, KC103, decompose?
T Questions 1-2: KC103 2 KCI+ 02 1. How many grams of O2 are produced from the decomposition of 8.56 grams of KC1032 2. How many grams of potassium chloride, KCl are produced if 13.2g of potassium chlorate, KC103, decompose?
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section: Chapter Questions
Problem 3.82QP
Related questions
Question
This is not a graded question to see how smart this app is, most of my friends get the question wrong

Transcribed Image Text:I Questions 1-2:
KC1O3 2
KCI+
O2
1. How many grams of O2 are produced from the decomposition of 8.56 grams of KC1032
2. How many grams of potassium chloride, KCl, are produced if 13.2g of potassium
chlorate, KC103, decompose?
66
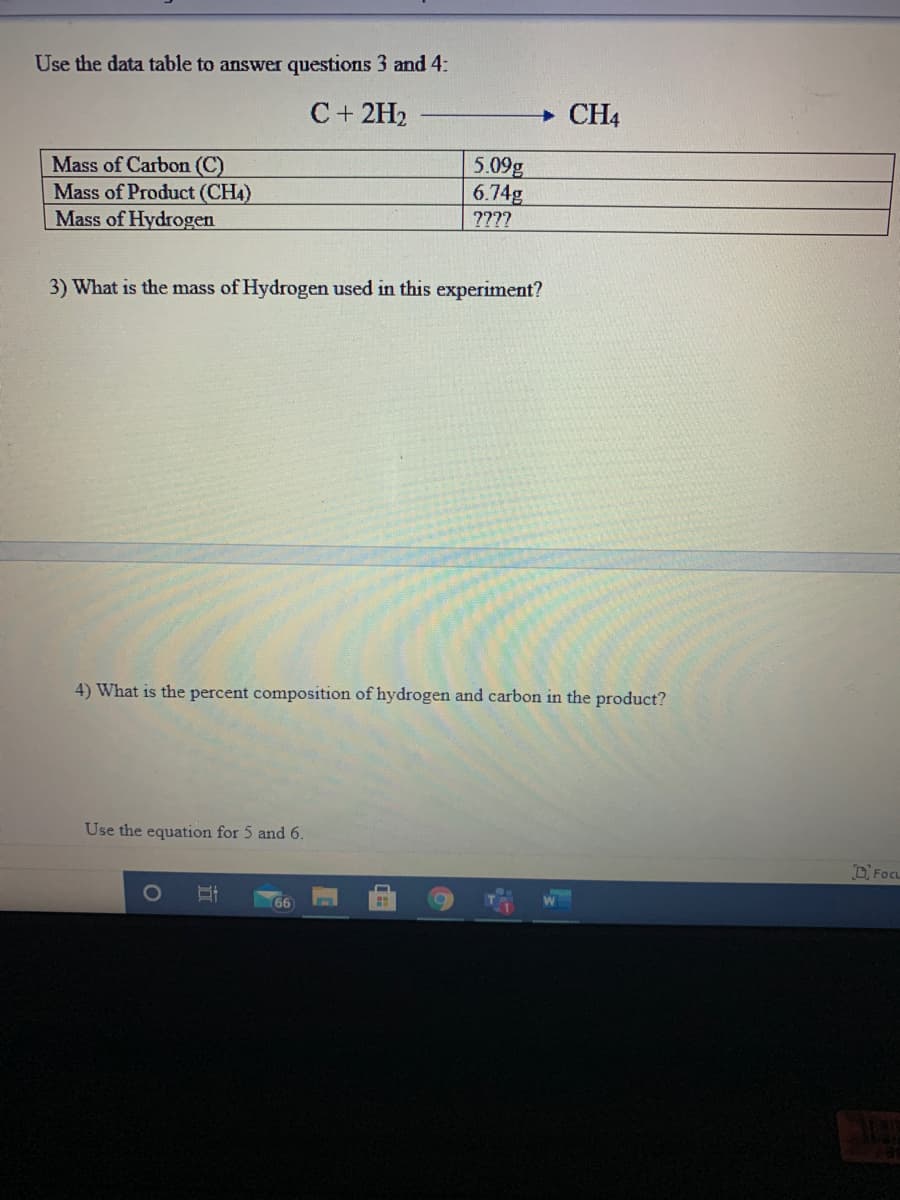
Transcribed Image Text:Use the data table to answer questions 3 and 4:
C+ 2H2
CH4
5.09g
6.74g
Mass of Carbon (C)
Mass of Product (CH4)
Mass of Hydrogen
????
3) What is the mass of Hydrogen used in this experiment?
4) What is the percent composition of hydrogen and carbon in the product?
Use the equation for 5 and 6.
D Focu
6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning