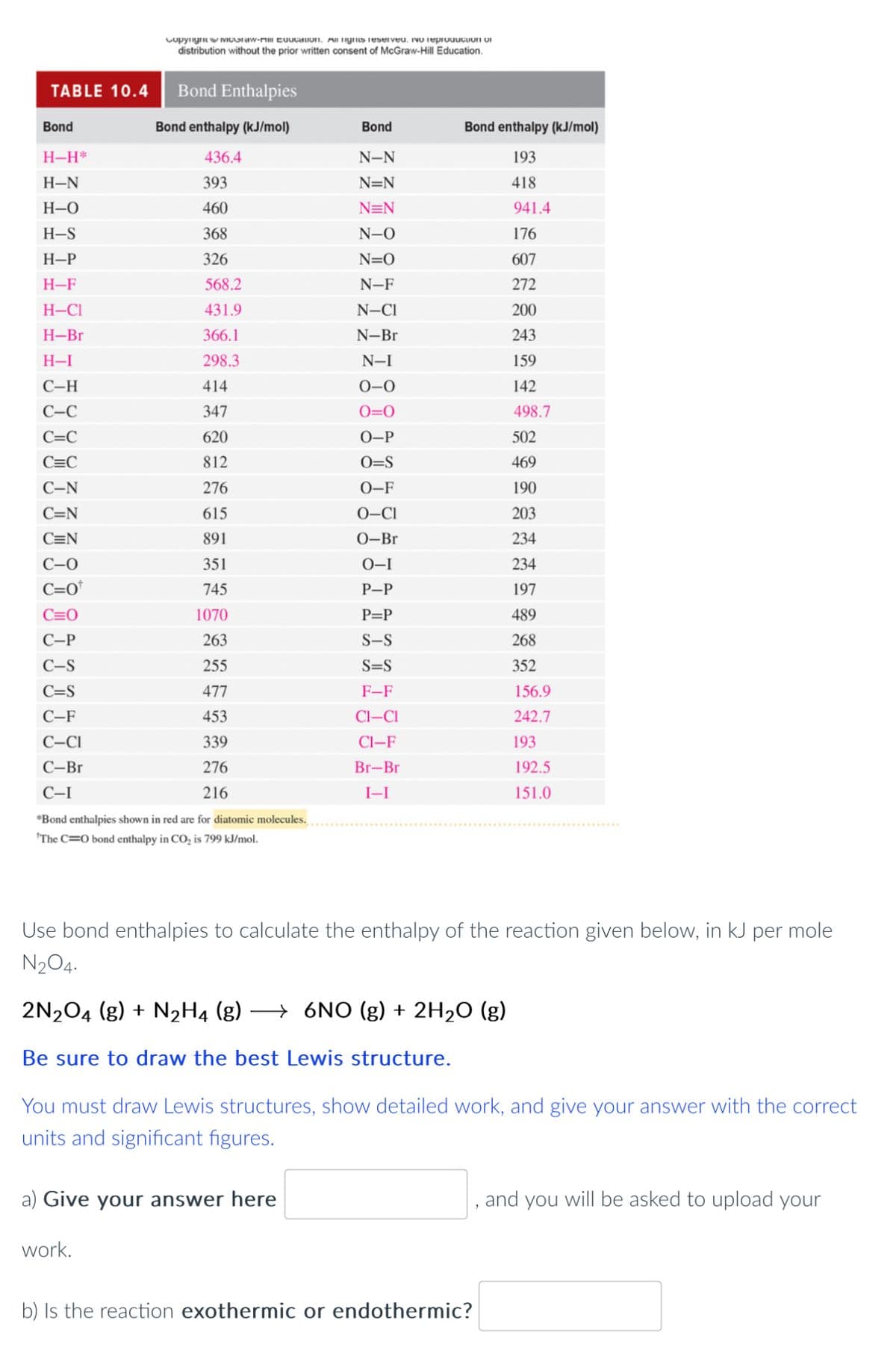
TABLE 10.4 Bond Enthalpies Bond enthalpy (kJ/mol) 436.4 393 460 368 326 Bond H-H* H-N H-O H-S H-P H-F H-CI H-Br H-I C-H C-C C=C C=C C-N C=N C=N C-0 C=0* C=0 C-P C-S C=S C-F C-Cl C-Br C-I Copyngoraw- cuucation. All rights reserved, no reproduction of distribution without the prior written consent of McGraw-Hill Education. 568.2 431.9 366.1 298.3 414 347 620 812 276 615 891 351 745 1070 263 255 477 453 339 276 216 *Bond enthalpies shown in red are for diatomic molecules. "The C=O bond enthalpy in CO₂ is 799 kJ/mol. work. a) Give your answer here Bond N-N N=N N=N N-O N=O N-F N-CI N-Br N-I 0-0 0=0 O-P O=S 0-F 0-CI 0-Br 0-1 P-P P=P S-S S=S F-F CI-CI CI-F Br-Br I-I Use bond enthalpies to calculate the enthalpy of the reaction given below, in kJ per mole N₂O4. 2N₂O4 (g) + N₂H4 (g) →6NO (g) + 2H₂O (g) Be sure to draw the best Lewis structure. You must draw Lewis structures, show detailed work, and give your answer with the correct units and significant figures. Bond enthalpy (kJ/mol) 193 418 941.4 176 607 272 200 243 159 142 498.7 502 469 190 203 234 234 197 489 268 352 156.9 242.7 193 192.5 151.0 b) Is the reaction exothermic or endothermic? , and you will be asked to upload your
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
I need help calculating the enthaply of this reaction please.
Step by step
Solved in 3 steps with 1 images




