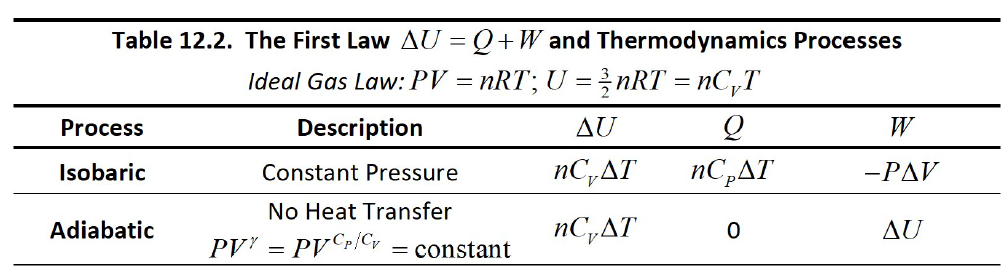
Table 12.2. The First Law AU = Q+W and Thermodynamics Processes Ideal Gas Law: PV = nRT; U =nRT = nC,T Process Description AU Isobaric пС,AТ ПСрАT -PAV Constant Pressure No Heat Transfer PV' = PV P/Cy = constant Adiabatic nC,AT AU
1.) Using the perfect
2.) If R=8.31 J/(K-mol), how many moles (n) are present in the cylinder?
3.) Assuming an adiabatic process (PVy = constant), calculate the value of the constant (y = 7/5).
4.) Assuming that the compression is done sufficiently fast that the constants (y and nR) do not change, calculate the pressure inside the cylinder after compression.
5.) Using the perfect gas law and the value found for nR in part a, calculate the temperature inside the cylinder after compression.
6.) How much work (assume CV = 5R/2) was accomplished during compression stroke (see entry for adiabatic processes in the table)?
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 8 images
