Table 14A.1 Interactions in macromolecules Interaction Potential energy Comment Bond stretching Vstretah (R) = } k, stretch(R – R? R, is the equilibrium bond length; ki stretch is the force constant Bond bending Voana() = 1 Kibana(0 – O,J? O, is the equilibrium bond angle; kibend is the force constant Vrorsion (o, y) = A(1 + cos 30) + B(1 + cos 3w) See Fig. 11; A and Bare constants of the order of 1 kJ mol-1 Bond torsion Partial charges Vcoulomb (r) = 4πει e is the permittivity of the medium C* C A Lennard-Jones (12,6) interaction (Topic 10B) Dispersion and repulsive interactions r12 ,6 D* V-bonding (r) – 12 Alternative to partial-charge interaction Hydrogen bonding r10
Table 14A.1 Interactions in macromolecules Interaction Potential energy Comment Bond stretching Vstretah (R) = } k, stretch(R – R? R, is the equilibrium bond length; ki stretch is the force constant Bond bending Voana() = 1 Kibana(0 – O,J? O, is the equilibrium bond angle; kibend is the force constant Vrorsion (o, y) = A(1 + cos 30) + B(1 + cos 3w) See Fig. 11; A and Bare constants of the order of 1 kJ mol-1 Bond torsion Partial charges Vcoulomb (r) = 4πει e is the permittivity of the medium C* C A Lennard-Jones (12,6) interaction (Topic 10B) Dispersion and repulsive interactions r12 ,6 D* V-bonding (r) – 12 Alternative to partial-charge interaction Hydrogen bonding r10
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter12: The Solid State
Section12.6: Phase Changes
Problem 2.1ACP
Related questions
Question
Use the appropriate equation in the given table to calculate the change in the potential energy when the angle of a C- C- C bond is increased by 5° from the equilibrium angle if the force constant is 0.62 aJ deg-2.
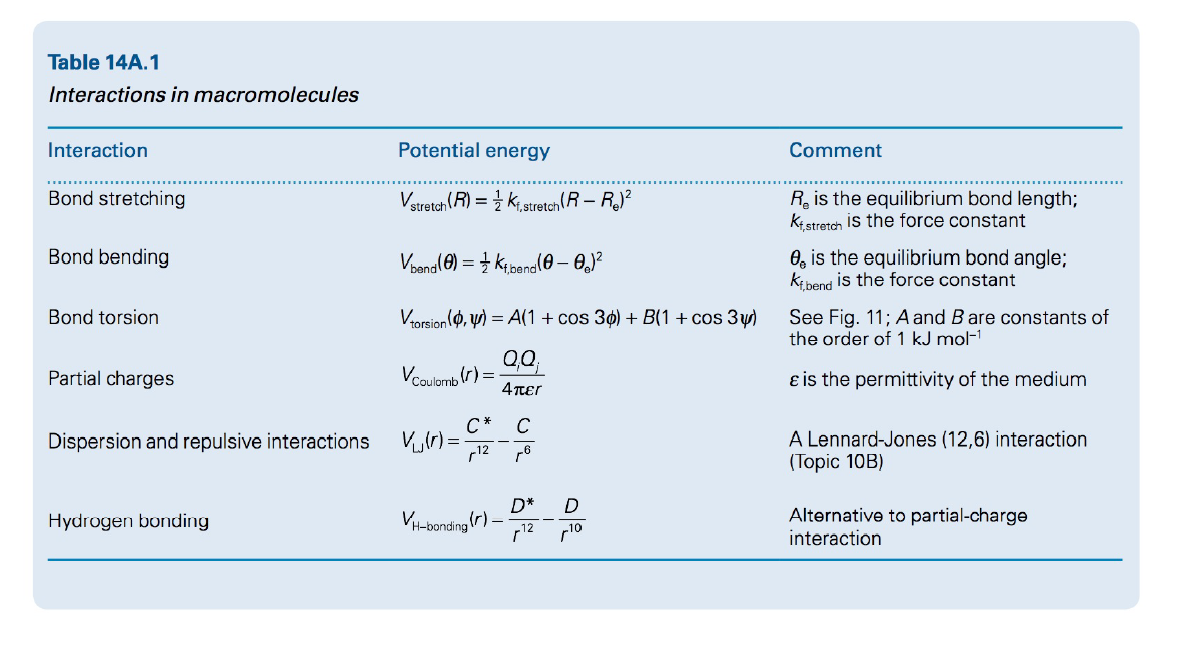
Transcribed Image Text:Table 14A.1
Interactions in macromolecules
Interaction
Potential energy
Comment
Bond stretching
Vstretah (R) = } k, stretch(R – R?
R, is the equilibrium bond length;
ki stretch is the force constant
Bond bending
Voana() = 1 Kibana(0 – O,J?
O, is the equilibrium bond angle;
kibend is the force constant
Vrorsion (o, y) = A(1 + cos 30) + B(1 + cos 3w)
See Fig. 11; A and Bare constants of
the order of 1 kJ mol-1
Bond torsion
Partial charges
Vcoulomb (r) =
4πει
e is the permittivity of the medium
C*
C
A Lennard-Jones (12,6) interaction
(Topic 10B)
Dispersion and repulsive interactions
r12 ,6
D*
V-bonding (r) –
12
Alternative to partial-charge
interaction
Hydrogen bonding
r10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning