Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter37: Qualitative Analysis Of Group Ii Cations
Section: Chapter Questions
Problem 3ASA
Related questions
Question
1. All answers to be entered via short answer are in the numerical format and presented up to the 3rd decimal place. Add trailing zeros if necessary, to complete your answer. NO NEED TO INCLUDE THE UNIT.
For uniformity of answers, please be guided by the following examples:
If your answer is 5.627594, enter 5.628
If your answer is 3.129934, enter 3.130
If your answer is 8.38, enter 8.380
If your answer is 1.2, enter 1.200
If your answer is 25, enter 25.000
If your answer is less than the value of 1, always place a zero before the decimal point
(example: 0.5, enter 0.500)
2. In cases for answers in the exponential format, please be guided by the following examples and format:
Do not convert your answer to decimal format
If your answer from the calculator is 1.23454566 x 10E-4, enter 1.235x10^-4 (no spaces in between)
If your answer from the calculator is 7.379566 x 10E-5, enter 7.38x10^-5 (no spaces in between)
3. For the atomic weights, it should be rounded to the nearest whole number.
Example:
Hydrogen: 1.00784 = 1.000
Based from the given problem, What is the molecular weight of potassium biphthalate (C8H5KO4)?
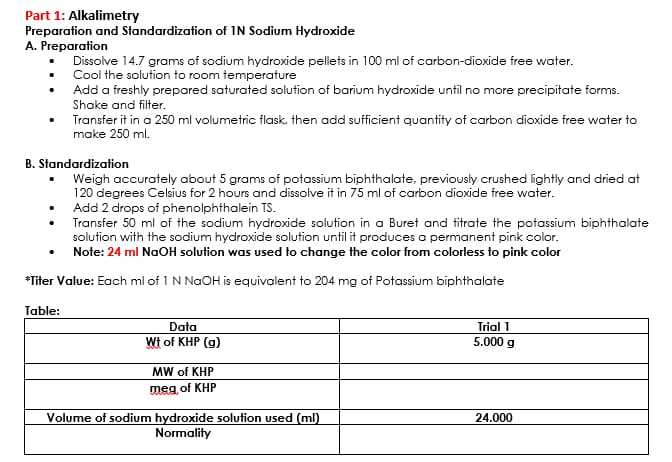
Transcribed Image Text:Part 1: Alkalimetry
Preparation and Standardization of IN Sodium Hydroxide
A. Preparation
Dissolve 14.7 grams of sodium hydroxide pellets in 100 ml of carbon-dioxide free water.
Cool the solution to room temperature
Add a freshly prepared saturated solution of barium hydroxide until no more precipitate forms.
Shake and filter.
Transfer it in a 250 ml volumetric flask, then add sufficient quantity of carbon dioxide free water to
make 250 ml.
B. Standardization
Weigh accurately about 5 grams of potassium biphthalate, previously crushed lightly and dried at
120 degrees Celsius for 2 hours and dissolve it in 75 ml of carbon dioxide free water.
Add 2 drops of phenolphthalein TS.
Transfer 50 ml of the sodium hydroxide solution in a Buret and titrate the potassium biphthalate
solution with the sodium hydroxide solution until it produces a permanent pink color.
Note: 24 ml NaOH solution was used to change the color from colorless to pink color
*Titer Value: Each ml of 1 N NaOH is equivalent to 204 mg of Potassium biphthalate
Table:
Data
Wt of KHP (g)
MW of KHP
meg of KHP
Volume of sodium hydroxide solution used (ml)
Normality
Trial 1
5.000 g
24.000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole
