TABLE ISummary of reuts of moecuar ougnostic tests ana tne rapia antigen tt for coviD-o usea on sef-conecta aasampes Total no. () of samples (95% confidence Interval) at the indicated time of colleetion since the onset of symptoms Test and primer set. method, or target Total no. (%) of samples (95% confidence Interval) (n Earty phase (S9 days) (n - 61) Late phase (9 days) (n 27) 103) No specifie time (asymptomatie) (n 15) RT-OPCR LDT 57 (934) (84.1-98 2) 17 (63.0) (424-80.6) 84 (01.6) (727-88 5) 10 166.7) (384-08.2) 54 (88.S) (778-95 2) 14 (S19) (319-71.3) 8 (53.3) (26.6-78.7) N-1 set 76 (738) (642-82.0) 83 (80 6) (716-877) 16 (59 3) (38.8-77.6) N-2 set 57 (934) (841-98 2) 10 (66.7) (384-88 2) cobas SARS-Cov2 test 83 (80 6) (716-877) 56 (918) (819-97.3) 18 (66.7) (46.0-835) 9 (60.0) (323-83.7) 54 (88 5) (778-95 2) 8 IS3.3 (26.6-78.7) Target 1 76 (73.8) (64 2-820) 14 (519) (319-713) Target 2 83 (80.6) (64.2-820) 56 (91.8) (819-973) 18 (667) (46.0-83.5) 9 160.0) (323-83.7) Direct RT-aPCR Method A 79 (76.7) (673-84 5) 53 (86 9) (758-94 2) 16 (593) (388-776) 10 (66.7) (384-88 2) e1 (78.6) (695-061) 55 (90 2) (798-96 3) 9 (60.0) (323-03.7) Method B 17 (63.0) (424-80 6) 80 (777) (684-0s3) 54 (88 5) (778-953 N-1 17 (63.0) (424-80.6) 9 (60.0) (323-83.7) set 48 (78.7) (66.3-88.) 8 (29 6 (13.8-50 2) N-2 63 161.2) (511-70.6) 7 (46.7) (213-73.4) set 52 (SO.5) (405-60.5) 6 (22.2) (8.6- 423) 6 (40.01 (16.3-67.7) Method c 40 (65.6) (523-773) 9 14 8 (70- 26.1) 15 (14.6) (8.4-229) 274) (10- 24.3) N-1 4 (26.7 (78-551) set N-2 51 (49 5) (39.5-59.5) 40 (65,6) 6 (22.21 (6.6- 5 (33.3) (18-616) RT-LAMP 73 (70.9) (611-79.4) 9 (60.0) (323-837) 52 (852) (73.8-93.0) 12 (44 4) (25.5-647) 8 (13.1) (5.8- 24 2) Rapid antigen test 12 1.7) (62-19.5) 2(74) (1.0- 24.3) 2 033) (17-405) LDT, iaboratory-developed test. Method A. SARS-Cov-2direct detection RT-aPCR iet TakaRa Bio inc, Kusatsu, Japan). Method B, Ampdirect 2o19 novel coronavirus detection kit (Snimadzu Corporation, Kyoto, Japan). Method C, SARS-Cov-2 detection kit Toyobo, Osaka, Japan). Primer and probe set recommended by the National institute of infectious Diseases (NID) in Japan Primer and probe set recommended by the Centers for Disease Control and Prevention (CDC) in the United States
TABLE ISummary of reuts of moecuar ougnostic tests ana tne rapia antigen tt for coviD-o usea on sef-conecta aasampes Total no. () of samples (95% confidence Interval) at the indicated time of colleetion since the onset of symptoms Test and primer set. method, or target Total no. (%) of samples (95% confidence Interval) (n Earty phase (S9 days) (n - 61) Late phase (9 days) (n 27) 103) No specifie time (asymptomatie) (n 15) RT-OPCR LDT 57 (934) (84.1-98 2) 17 (63.0) (424-80.6) 84 (01.6) (727-88 5) 10 166.7) (384-08.2) 54 (88.S) (778-95 2) 14 (S19) (319-71.3) 8 (53.3) (26.6-78.7) N-1 set 76 (738) (642-82.0) 83 (80 6) (716-877) 16 (59 3) (38.8-77.6) N-2 set 57 (934) (841-98 2) 10 (66.7) (384-88 2) cobas SARS-Cov2 test 83 (80 6) (716-877) 56 (918) (819-97.3) 18 (66.7) (46.0-835) 9 (60.0) (323-83.7) 54 (88 5) (778-95 2) 8 IS3.3 (26.6-78.7) Target 1 76 (73.8) (64 2-820) 14 (519) (319-713) Target 2 83 (80.6) (64.2-820) 56 (91.8) (819-973) 18 (667) (46.0-83.5) 9 160.0) (323-83.7) Direct RT-aPCR Method A 79 (76.7) (673-84 5) 53 (86 9) (758-94 2) 16 (593) (388-776) 10 (66.7) (384-88 2) e1 (78.6) (695-061) 55 (90 2) (798-96 3) 9 (60.0) (323-03.7) Method B 17 (63.0) (424-80 6) 80 (777) (684-0s3) 54 (88 5) (778-953 N-1 17 (63.0) (424-80.6) 9 (60.0) (323-83.7) set 48 (78.7) (66.3-88.) 8 (29 6 (13.8-50 2) N-2 63 161.2) (511-70.6) 7 (46.7) (213-73.4) set 52 (SO.5) (405-60.5) 6 (22.2) (8.6- 423) 6 (40.01 (16.3-67.7) Method c 40 (65.6) (523-773) 9 14 8 (70- 26.1) 15 (14.6) (8.4-229) 274) (10- 24.3) N-1 4 (26.7 (78-551) set N-2 51 (49 5) (39.5-59.5) 40 (65,6) 6 (22.21 (6.6- 5 (33.3) (18-616) RT-LAMP 73 (70.9) (611-79.4) 9 (60.0) (323-837) 52 (852) (73.8-93.0) 12 (44 4) (25.5-647) 8 (13.1) (5.8- 24 2) Rapid antigen test 12 1.7) (62-19.5) 2(74) (1.0- 24.3) 2 033) (17-405) LDT, iaboratory-developed test. Method A. SARS-Cov-2direct detection RT-aPCR iet TakaRa Bio inc, Kusatsu, Japan). Method B, Ampdirect 2o19 novel coronavirus detection kit (Snimadzu Corporation, Kyoto, Japan). Method C, SARS-Cov-2 detection kit Toyobo, Osaka, Japan). Primer and probe set recommended by the National institute of infectious Diseases (NID) in Japan Primer and probe set recommended by the Centers for Disease Control and Prevention (CDC) in the United States
A First Course in Probability (10th Edition)
10th Edition
ISBN:9780134753119
Author:Sheldon Ross
Publisher:Sheldon Ross
Chapter1: Combinatorial Analysis
Section: Chapter Questions
Problem 1.1P: a. How many different 7-place license plates are possible if the first 2 places are for letters and...
Related questions
Question
Describe in detail based on the result above
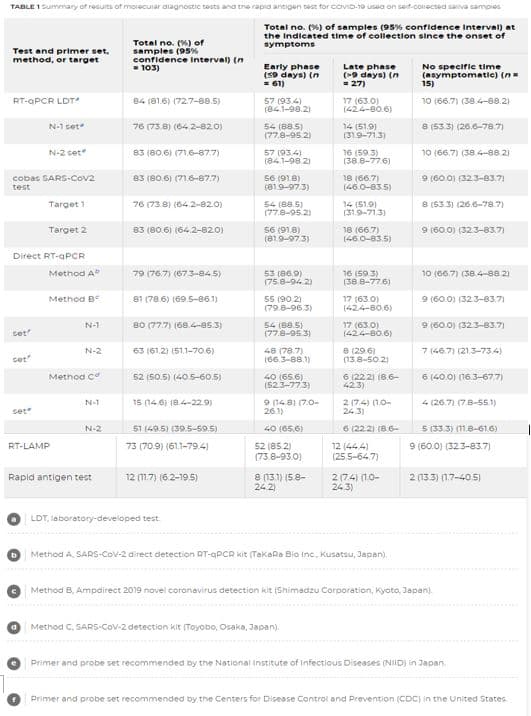
Transcribed Image Text:TABLE 1Summary of reciurts of morecuiar alagnostic tests ana tne rapia antigen tast for covID-19 usea on seif-comectea sava sampes
Total no. (%) of samples (95% confidence Interval) at
the Indicated time of collection since the onset of
symptoms
Total no. (%) of
samples (95%6
confidence Interval) (n
- 103)
Test and primer set,
method, or target
Early phase
(S9 days) (n
- 61)
Late phase
(-9 days) (n
= 27)
No specific time
(asymptomatic) (n=
15)
RT-GPCR LDT
57 (93.4)
(84.1-98.2)
17 (63.0)
(424-80.6)
84 (81.6) (727-88.)
10 (66.7) (38.4-88.2)
76 (73.8) (64.2-82.0)
54 (88 5)
(77.8-95 2)
14 (51.9)
(31.9-71.3)
8 (53.3) (26.6-78.7)
N-1 set
N-2 set
83 (80.6) (71,6-87.7)
57 (93 4)
(84.1-98.2)
16 (59 3)
(38.8-77.6)
10 (66,7) (38.-88.2)
56 (91.8)
(81.9-97.3)
9 (60.0) (323-83.7)
cobas SARS-Cov2
83 (80.6) (71.6-877)
18 (66.7)
(46.0-83.5)
test
Target 1
76 (73.8) (64.2-82.0)
54 (88.5)
(77.8-95.2)
14 (51.9)
(31.9-71.3)
8 (53.31 (26.6-78.7)
56 (91.8)
(819-97.3)
18 (66.7)
(46.0-83.5)
9 (60.0) (323-83.7)
Target 2
83 (B0.6) (64.2-82.0)
Direct RT-aPCR
Method A
79 (76.7) (673-84 5)
53 (86.9)
(758-94 2)
16 (59 3)
(38.8-77.6)
10 (66.7) (38.4-88.2)
81 (78.6) (69.5-861)
55 (90.2)
(79.8-96 3)
17 (63.0)
(424-80.6)
9 (60.0) (323-83.7)
Method B
80 (77.7) (68.4-85.3)
54 (88.5)
(77.8-95.3)
9 (60.0) (323-83.7)
N-1
17 (63.0)
(424-80.6)
set
63 (61.2) (51.1-706)
48 (78.7)
(66.3-88.1)
8 (29 6)
(13.8-50.21
7 (46.7) (21.3-734)
N-2
52 (S0.5) (405-60.5)
6 (22 2) (8.6-
423)
Method co
40 (65.6)
(523-773)
6 (40.0) (16.3-67.7)
9 (14.8) (7.0-
26.1)
15 (14.6) (8.4-22.9)
2 (7.4) (1.0-
24.3)
N-1
4 (26.7) (7.8-5.1)
set
N-2
51 (49.5) (39.5-59.5)
40 (65,6)
6 (22 2) (8.6-
5 (33.3) (11.8-61.6)
12 (44.4)
(25.5-64.7)
RT-LAMP
73 (70.9) (61.1-79.4)
9 (60.0) (323-83.7)
52 (85.2)
(73.8-93.0)
8 131) (5.8-
242)
Rapid antigen test
12 (1.7) (6.2-19.5)
2 (13.3) (1.7-40.5)
2 (74) (1.0-
24.3)
LDT, laboratory-developed test.
Method A, SARS-Cov-2 direct detection RT-qPCR kit (TakaRa Bio Inc, Kusatsu, Japan).
Method B, Ampdirect 2019 novel coronavirus detection kit (Shimadzu Corporation, Kyoto, Japan).
Method C, SARS-COV-2 detection kit (Toyobo, Osaka, Japan).
Primer and probe set recommended by the National Institute of infectious Diseases (NIID) in Japan
Primer and probe set recommended by the Centers for Disease Control and Prevention (CDC) in the United States
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

A First Course in Probability (10th Edition)
Probability
ISBN:
9780134753119
Author:
Sheldon Ross
Publisher:
PEARSON


A First Course in Probability (10th Edition)
Probability
ISBN:
9780134753119
Author:
Sheldon Ross
Publisher:
PEARSON
